- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Capreomycin
Capreomycin: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. In case of impaired renal function
- 12. Use in the elderly
- 13. Drug interactions
- 14. Analogs
- 15. Terms and conditions of storage
- 16. Terms of dispensing from pharmacies
- 17. Reviews
- 18. Price in pharmacies
Latin name: Capreomycin
ATX code: J04AB30
Active ingredient: capreomycin (capreomycinum)
Manufacturer: Sanjivani Paranteral Limited (India), PharmConcept, LLC (Russia), Lok-Beta Pharmaceuticals (I) Pvt. Ltd (India), Belko Pharma (India), Krasfarma, OJSC (Russia), Sintez, OJSC (Russia)
Description and photo update: 10.07.2018
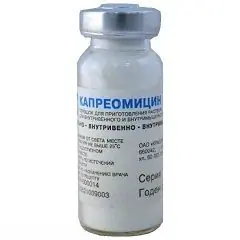
Capreomycin is an antibacterial drug for the treatment of pulmonary tuberculosis.
Release form and composition
The dosage form is a powder for the preparation of a solution for intravenous and intramuscular administration: white or almost white (500, 750 and 1000 mg each in 10 ml vials, hermetically sealed with rubber stoppers, which are crimped with aluminum caps or combined aluminum caps with plastic caps, in a cardboard box 1, 5 or 10 bottles; packaging for hospitals - 50 bottles in a cardboard box).
The active substance in one bottle: capreomycin sulfate - 500, 750 or 1000 mg in terms of capreomycin.
Pharmacological properties
Pharmacodynamics
Capreomycin is a polypeptide antibiotic produced by bacteria of the species Streptomyces capreolus. It has a bacteriostatic effect only on strains of Mycobacterium tuberculosis (when determined in a liquid medium, the minimum inhibitory concentration averages 1.25-2.5 mg / l).
The mechanism of action of the drug is due to its ability to suppress protein synthesis in a bacterial cell.
According to the classification of the World Health Organization, capreomycin is a second-line anti-tuberculosis drug.
With monotherapy, capreomycin quickly causes the development of resistant strains of mycobacteria, therefore it is used only as part of complex therapy as a second-line anti-tuberculosis drug, including in cases of ineffectiveness and intolerance of first-line anti-tuberculosis drugs (such as pyrazinamide, ethambutol, rifampicin, streptomycin, isoniazid), and also in the presence of sensitivity of mycobacteria to capreomycin and simultaneously used other anti-tuberculosis drugs.
Capreomycin has full cross-resistance with viomycin, partial cross-resistance with several aminoglycoside antibiotics (including neomycin and kanamycin). Cross-resistance is not observed between capreomycin and aminosalicylic acid, streptomycin, ethambutol, cycloserine, isoniazid, ethionamide.
The drug has a teratogenic effect. In experiments on rats, skeletal anomalies were revealed.
Pharmacokinetics
Capreomycin is injected into the body only parenterally, since it is not absorbed in the gastrointestinal tract. After intramuscular (i / m) injection of 1000 mg of the drug, the maximum concentration in the blood plasma is reached after 1-2 hours and is 20-47 mg / l, after 10 hours the concentration is approximately 4 mg / l. After intravenous (iv) administration of 1000 mg of the drug, the maximum plasma concentration is reached within 1 hour and is 30-50 mg / l.
The AUC (area under the concentration-time curve) is the same for intramuscular and intravenous administration.
The drug is excreted mainly by the kidneys (within 12 hours, approximately 50-60% of the administered dose) by glomerular filtration unchanged, in small amounts - with bile.
Capreomycin crosses the placental barrier. Does not penetrate the blood-brain barrier. Does not accumulate in the body when administered daily at a dose of 1000 mg for 30 days, provided that the renal function is normal. In patients with impaired renal function, the half-life of the drug increases, and a tendency to cumulation is noted.
Indications for use
According to the instructions, Capreomycin is used as part of the complex therapy of pulmonary tuberculosis in patients with resistance to anti-tuberculosis drugs of the first line or their intolerance.
Contraindications
- childhood;
- period of pregnancy and lactation;
- hypersensitivity to the drug.
The drug is used with caution:
- with renal failure;
- with hearing impairment;
- with allergies (including drug);
- with myasthenia gravis;
- with dehydration;
- with parkinsonism;
- in old age;
- with simultaneous use with drugs that cause neuromuscular blockade (especially with a high risk of its complete cessation), during and after surgery.
Instructions for the use of Capreomycin: method and dosage
Before starting therapy, the sensitivity of the Mycobacterium tuberculosis strain to capreomycin should be checked.
The drug is used in combination with other anti-tuberculosis drugs. It is injected deeply intramuscularly or intravenously in the form of a long (60 minutes) drip infusion, usually 1000 mg once a day for 60-120 days, then 1000 mg 2-3 times a week for 12-24 months.
The optimal daily dose is determined individually; it should not exceed 20 mg / kg.
The dose of capreomycin for patients with impaired renal function depends on creatinine clearance (CC). Creatinine clearance - dose for dosing intervals 24/48/72 hours:
- CC 0 - 1.29 / 2.58 / 3.87 mg / kg;
- CC 10 - 2.43 / 4.87 / 7.30 mg / kg;
- KK 20 - 3.58 / 7.16 / 10.7 mg / kg;
- CC 30 - 4.72 / 9.45 / 14.2 mg / kg;
- CC 40 - 5.87 / 11.7 mg / kg;
- KK 50 - 7.01 / 14 mg / kg;
- KK 60 - 8.16 mg / kg;
- CC 80 - 10.4 mg / kg;
- CC 100 - 12.7 mg / kg;
- CC 110 - 13.9 mg / kg.
For intramuscular administration, the contents of one vial are dissolved in sterile water for injection or sodium chloride solution 0.9%: 500 mg in 1 ml, 750 mg in 1.5 ml, 1000 mg in 2 ml. Wait 2-3 minutes until the powder is completely dissolved. The drug should be injected deep into the muscle. Superficial injection is usually painful and may cause an aseptic abscess.
For intravenous administration, the solution prepared by the above method is diluted with 100 ml of 0.9% sodium chloride solution, injected within 60 minutes.
For a 1000 mg dose, use the entire vial containing 1000 mg of capreomycin. If it is necessary to administer a lower dose, it is recommended to use the following data for dilution. The volume of solution and its concentration depend on the amount of solvent added to a 10 ml vial containing a dose of 1000 mg:
- 2.15 ml of solvent: 2.85 ml of a solution with a concentration of 370 mg / ml;
- 2.63 ml of solvent: 3.33 ml of a solution with a concentration of 315 mg / ml;
- 3.3 ml solvent: 4 ml 260 mg / ml solution;
- 4.3 ml solvent: 5 ml 210 mg / ml solution.
The solution prepared from the powder may acquire a pale straw color and darken over time. This phenomenon does not affect the effectiveness of the drug and its toxicity. The diluted solution can be stored in the refrigerator for 1 day.
Side effects
- from the central nervous system: neuromuscular blockade, neurotoxicity;
- from the liver: with the simultaneous use of other anti-tuberculosis drugs that cause changes in liver function - impaired liver function indicators;
- from the hematopoietic system: hematuria, cylindruria, leukocyturia, hyperuricuria, leukopenia, leukocytosis, eosinophilia, thrombocytopenia;
- on the part of the urinary system: nephrotoxicity, including electrolyte imbalance (reminiscent of the development of toxic nephritis and Barter's syndrome), abnormal urinary sediment, a decrease in the excretion of phenolsulfonphthalein, an increase in the level of urea nitrogen in the blood of more than 20 mg / 100 ml;
- from the senses: ototoxicity (dizziness, tinnitus, subclinical hearing loss);
- local reactions: pain, induration and increased bleeding at the injection site, aseptic abscess;
- allergic reactions: with the simultaneous use of anti-tuberculosis drugs - urticaria and skin rashes in the form of spots and nodules, sometimes accompanied by fever.
Overdose
Symptoms: decreased general tone, damage to the auditory and vestibular parts of the VII pair of cranial nerves (tinnitus, vertigo, dizziness), electrolyte imbalance, hypocalcemia, hypokalemia, hypomagnesemia, neuromuscular blockade (respiratory paralysis), acute renal tubular necrosis.
Patients with normal renal function are shown hydration with maintenance of urination at the level of 3-5 ml / h / kg, control and, if necessary, correction of creatinine clearance, electrolyte levels and water balance. To eliminate the neuromuscular blockade, calcium preparations and cholinesterase inhibitors are administered.
Hemodialysis is indicated for patients with severe renal impairment.
special instructions
Before the appointment of Capreomycin and regularly during its use (1-2 times a week) audiometry and assessment of vestibular function are required.
Also, before prescribing the drug and once a week during the period of its use, it is necessary to monitor the concentration of capreomycin in the blood, hematological parameters and liver function.
With the use of the drug, kidney damage is possible, accompanied by the appearance of a pathological sediment in the urine, an increase in the level of urea nitrogen in the blood or serum creatinine, and necrosis of the renal tubules. In the elderly, patients with dehydration, impaired renal function, and patients receiving other nephrotoxic drugs, the risk of acute renal tubular necrosis increases significantly.
In a large number of patients taking the drug for a long time, there is a slight increase in serum creatinine and urea nitrogen. In many of them, the appearance of erythrocytes, leukocytes and casts in the urine was noted. In case of an increase in the level of urea nitrogen by more than 30 mg / 100 ml or the appearance of any sign of decreased renal function (including with an increase in the level of urea nitrogen), a careful examination of the patient is required, a decrease in the dose of Capreomycin depending on the level of CC or a complete cessation of therapy.
Against the background of drug therapy, damage to the auditory and vestibular parts of the VIII pair of cranial nerves is possible. Most often, this disorder is detected in patients with dehydration or impaired renal function and patients receiving simultaneously drugs with ototoxic action. The most common symptoms of this injury are tinnitus and dizziness.
In the case of a rapid intravenous injection of the drug, there is a possibility of developing a neuromuscular blockade or respiratory paralysis.
Capreomycin can cause hypokalemia, therefore, it is recommended to determine the serum potassium content once a month during the entire period of therapy.
During treatment, it is necessary to constantly monitor the dosage regimens and regimen, as well as the regularity and correctness of prescriptions. If the next injection is missed, the next one should be administered as soon as possible, only if it is not time for the next dose. The use of a double dose is prohibited.
It is not recommended to use viomycin and streptomycin during the treatment with capreomycin.
Influence on the ability to drive vehicles and complex mechanisms
Care should be taken when driving and performing potentially hazardous types of work that require increased attention and speed of reactions.
Application during pregnancy and lactation
Capreomycin is contraindicated during pregnancy and lactation.
If treatment is required during lactation, breastfeeding should be discontinued.
Pediatric use
The drug is not used in pediatrics.
With impaired renal function
In renal failure, Capreomycin can be prescribed only after a careful assessment of the ratio of the expected benefit and the possible risk of kidney damage. The dose of the drug is reduced in accordance with the level of creatinine clearance.
Use in the elderly
Capreomycin should be used with caution in the treatment of elderly patients.
Drug interactions
Aminoglycosides, polymyxins, citrate blood preservatives and diethyl ether enhance the muscle relaxant effect, while neostigmine methyl sulfate reduces it.
Mutual enhancement of nephrotoxic and ototoxic action is possible with the simultaneous use of capreomycin with other anti-tuberculosis drugs (viomycin, streptomycin), as well as in combination with ethacrynic acid, gentamycin, furosemide, sodium colistimethate, kanamycin, methoxyflurane, tobramyxincomycin, polymycin.
Analogs
Capreomycin analogs are Capastat, Capocin, Capremabol, Capreomycin-DECO, Capreomycin sulfate, Capreomycin-Ferein, Laikocin, Capreostat.
Terms and conditions of storage
Store out of the reach of children, protected from light and moisture, at temperatures up to 25 ° C.
The shelf life is 3 years.
Terms of dispensing from pharmacies
Dispensed by prescription.
Reviews of Capreomycin
On specialized medical sites, there are no reviews about Capreomycin, which would allow assessing its effectiveness and safety of use.
The price of Capreomycin in pharmacies
The approximate price for Capreomycin is 506-990 rubles. for 1 bottle containing the drug in a dose of 1000 mg.

Maria Kulkes Medical journalist About the author
Education: First Moscow State Medical University named after I. M. Sechenov, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!