- Author Rachel Wainwright [email protected].
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
ADASEL
ADASEL: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. Use in the elderly
- 12. Drug interactions
- 13. Analogs
- 14. Terms and conditions of storage
- 15. Terms of dispensing from pharmacies
- 16. Reviews
- 17. Price in pharmacies
Latin name: ADACEL
ATX code: J07AJ52
Active ingredient: vaccine for the prevention of diphtheria (with reduced antigen content), tetanus and pertussis (acellular), combined, adsorbed (tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine, adsorbed)
Producer: Sanofi Pasteur Limited (Canada)
Description and photo updated: 2018-29-11
Prices in pharmacies: from 1990 rubles.
Buy
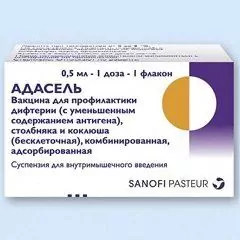
ADASEL [Vaccine for the prevention of diphtheria (with reduced antigen content), tetanus and pertussis (acellular), combined, adsorbed] - MIBP (medical immunobiological drug) for the prevention of diphtheria, tetanus, whooping cough.
Release form and composition
Dosage form - suspension for intramuscular administration: turbid, whitish, homogeneous liquid (in a cardboard box 1 or 5 vials of 2 ml, containing 1 dose of 0.5 ml vaccine, and instructions for use of ADASEL).
Active substances in 1 dose (0.5 ml):
- adsorbed tetanus toxoid - 5 Lf * [more than 20 IU (international units)];
- adsorbed diphtheria toxoid - 2 Lf (more than 2 IU);
- acellular pertussis vaccine containing: adsorbed filamentous hemagglutinin - 5 μg; adsorbed pertussis toxoid - 2.5 μg; adsorbed pertactin - 3 μg; adsorbed agglutinogens (fimbria) of types 2 and 3 - 5 μg.
Auxiliary components: aluminum (in the form of aluminum phosphate) - 0.000 33 μg; 2-phenoxyethanol - 0.6% (total); water for injection - up to 0.5 ml.
* Lf - Limes flocculationis (flocculation threshold), the amount of toxoid entering into the flocculation reaction with 1 unit of antitoxin.
Pharmacological properties
Pharmacodynamics
Tetanus
The mechanism of protection against tetanus is associated with the production of neutralizing antibodies to tetanus toxin. In this case, the lowest protective serum concentration of tetanus antitoxic antibodies, which is determined using the neutralization method, should be at least 0.01 IU / ml.
In clinical studies of ADASEL, the protective concentration of tetanus antitoxic antibodies was determined as 0.1 IU / ml, and an increase in the concentration of antitoxic antibodies to 1.0 IU / ml is associated with long-term protection. The measurements were carried out using the ELISA method (Enzyme Linked Immunoadsorbent Assay) - determination using immunosorbents associated with enzymes.
After vaccine administration, the immune response to tetanus toxin reaches a level previously defined as protective (≥ 0.1 IU / ml). This confirms the immunological efficacy of tetanus toxoid used in ADASEL.
Diphtheria
The defense mechanism against diphtheria is provided by antibodies that neutralize the diphtheria toxin. The lowest serum concentration of anti-diphtheria antitoxic antibodies that protects against disease is 0.01 IU / ml.
An anti-diphtheria antibody concentration ≥ 0.1 IU / ml is considered protective, and ≥ 1.0 IU / ml is considered a long-term protection.
After administration of ADASEL, the immune response to diphtheria toxin reaches the level previously defined as protective (≥ 0.1 IU / ml). This confirms the immunological effectiveness of the diphtheria toxoid used in the vaccine.
Whooping cough
The effectiveness of the pertussis antigens that make up ADASEL was confirmed by comparing the concentration of antibodies to these antigens, which were achieved in single-dose ADASEL, with the concentration of antibodies to the same antigens, which were achieved in children under one year old who received three-fold immunization with the vaccine for children under 12 months. containing tetanus toxoid, AbCDS (a similar acellular pertussis component) and diphtheria toxoid (comparison was carried out in the study of the epidemiological effectiveness of ADASEL, conducted in 1992-1995 in Sweden - Sweden I).
The value of the indicator of the epidemiological effectiveness of ABCDS is 84.9% against confirmed whooping cough (a disease with bouts of convulsive cough lasting at least 21 days with the release of the pathogen Bordetella pertussis or with an established epidemiological connection with a laboratory-confirmed case of the disease). And the epidemiological effectiveness of the vaccine in relation to whooping cough, which is mild (at least one day with coughing fits, with the release of Bordetella pertussis) is 77.9%.
The acellular pertussis components that make up ADASEL and ABCD differ only in the number of CAs (in ADASEL - 2.5 μg, in ABCD - 10 μg). When conducting clinical studies of the humoral response to pertussis antigens in adults and children, it was found that revaccination with one dose of ADASEL leads to pronounced formation of antibodies to all pertussis antigens included in the vaccine, while the post-vaccination level of antibodies is 2-5 times higher than the level protection registered in the Sweden I.
The efficacy of vaccines for the prevention of tetanus, diphtheria (low antigen) and pertussis (acellular) (AdSbq), including ADASEL, used to prevent pertussis, has been confirmed in various studies. In adolescents who were immunized against whooping cough with the whole-cell vaccine during infancy / early childhood, the effectiveness of the AdSbq vaccines during pertussis outbreaks was in the 66-75% range. Similarly, in adolescents who received primary immunization with acellular pertussis vaccine, the effectiveness of AdSbq vaccines during pertussis outbreaks during the first year after vaccination ranged from 73 to 75%.
Immunogenicity in different age groups of patients
The following groups of patients took part in comparative studies: children 4-6 years old; adolescents 11-17 years old; adults 18-64 years old.
The concentration of antibodies in these studies was determined one month (28-35 days) after the application of ADASEL.
It was found:
- tetanus: in 100% of cases of vaccine use in all groups of patients, the concentration of antibodies against tetanus toxoid at the level of protection (≥ 0.1 U / ml) was achieved;
- diphtheria: protective concentration of antibodies against diphtheria toxoid (≥ 0.1 U / ml) was observed in 94.1% of adults, 99.8% of adolescents and 100% of children;
- pertussis: when assessing the level of the immune response to pertussis antigens in all age groups of patients, it was noted that revaccination leads to a pronounced increase in the level of antitoxic antibodies against pertussis toxin, which exceeds the protective level observed in the Sweden I study by 2-5 times.
Duration of protective action ADASEL
During a long-term (over 10 years) observation of the persistence of the level of antibodies to vaccine antigens in adults and adolescents who were vaccinated in early childhood against tetanus, diphtheria and whooping cough (using a vaccine that included a whole-cell pertussis component) and once revaccinated ADASEL, it was demonstrated that the protective indicators for tetanus and diphtheria toxoids are preserved 10 years after vaccination (respectively, in 99.2 and 92.6%).
The concentration of pertussis antibodies for 5 years remained 2-9 times higher than the initial value, 10 years after vaccination, this level decreased to the initial (before vaccination). Studies of the duration of post-vaccination immunity and data on the study of repeated administrations of ADASEL confirm the possibility of its use with an interval of 10 years instead of vaccines that contain only diphtheria and tetanus toxoids.
Indications for use
The ADASEL vaccine is used in persons 4-64 years of age for the purpose of revaccination against tetanus, diphtheria and whooping cough.
Contraindications
Absolute:
- children under 4 years old, elderly over 64 years old;
- encephalopathy (for example, impaired consciousness, coma, repeated convulsions) within seven days after the administration of the vaccine, which included the pertussis component, unless another cause is established;
- progressive neurological disease, progressive encephalopathy, or uncontrolled epilepsy;
- acute diseases of infectious / non-infectious etiology, exacerbation of chronic diseases (are temporary contraindications; in this case, vaccination can be carried out after recovery or during remission). In case of acute intestinal diseases, mild acute respiratory viral infections and other conditions, vaccination is possible immediately after normalization of body temperature;
- burdened history of anaphylactic reactions to drugs containing diphtheria, tetanus toxoid and pertussis vaccine.
Relative (ADASEL is prescribed under medical supervision):
- pregnancy;
- lactation period.
ADASEL, instructions for use: method and dosage
The ADASEL vaccine is injected intramuscularly into the deltoid muscle of the shoulder. You can not inject the vaccine intra- or subcutaneously, into the gluteus muscle and the vascular bed.
The dose of ADASEL for revaccination is 0.5 ml once.
Based on national guidelines, if necessary, ADASEL can be used in adults and older children instead of diphtheria and tetanus vaccine for booster vaccinations against diphtheria, pertussis and tetanus.
In the treatment room, where immunization is carried out, there should be anti-shock therapy (epinephrine hydrochloride solution for injection 1 ÷ 1000, glucocorticosteroids and other appropriate drugs). The recommended duration of observation of the patient's condition after drug administration is at least 30 minutes.
Before the introduction of ADASEL, the contents of the vial should be assessed for discoloration and / or the presence of foreign inclusions. If any abnormalities are found, the vaccine cannot be administered.
Before administration, shake the bottle until a turbid homogeneous suspension is obtained.
The cork of the vial must be disinfected with an antiseptic before taking the vaccine dose.
The stopper and the metal cap that holds it should not be removed from the bottle.
The introduction of the vaccine should be carried out in compliance with the rules of asepsis.
Side effects
Pain at the injection site, according to the results of clinical studies of ADASEL in persons aged 4-64 years, is the most common local reaction caused by the injection method of drug administration.
In most cases, the development of local reactions associated with the introduction of the suspension was noted within three days from the moment of vaccination, their average duration is less than 3 days.
Erythema / edema at the injection site of ≥ 35 mm 3 days after vaccination was noted (respectively):
- children: 11.7 / 10.1%
- adolescents: 5.9 / 6.2%
- adults: 4.8 / 5.2%
The most common general reactions: in children - increased fatigue, in adolescents and adults - headache. An increase in body temperature> 38 ° C was noted in less than 10% of cases. These disturbances were short-term and characterized by mild or moderate intensity. An increase in body temperature> 39.5 ° C three days after vaccination was observed in children and adolescents (in 0.3 and 0.1% of cases) and was not recorded in adults.
Possible adverse reactions (> 10% - very common;> 1% and 0.1% and 0.01% and <0.1% - rarely; <0.01% - very rare):
- systemic reactions (1 - children, 2 - adolescents, 3 - adults): very often - swelling in the joints 2,3, headache, nausea 2, anorexia 1, diarrhea, myalgia 2,3, muscle pain or muscle weakness 2, 3, general malaise, chills 2; often - nausea 1, fever, vomiting, myalgia 1, enlarged axillary lymph nodes, skin rash, muscle pain or muscle weakness 1, chills 1.3, swelling in the joints 1;
- local reactions at the injection site: very often - swelling, pain, redness.
Information about the following adverse events was received as spontaneous messages during the post-marketing period of ADASEL use, while it is impossible to assess their causal relationship with the use of the vaccine and the frequency of occurrence.
Possible violations recorded during the post-registration use of the vaccine:
- nervous system: fainting, hypesthesia, Guillain-Barré syndrome, paresthesia, brachial nerve neuritis, facial paralysis, convulsions, myelitis;
- immune system: hypersensitivity reaction (anaphylactic) - rash, edema, angioedema, hypotension;
- cardiovascular system: myocarditis;
- musculoskeletal system: muscle cramps, myositis;
- skin and subcutaneous tissue: rashes, itching;
- reactions at the vaccine injection site and general responses: aseptic abscess, hematoma at the injection site, widespread reactions at the injection site (more than 50 mm), extensive swelling of the limb that spreads from the ADASEL injection site beyond one / two joints.
Overdose
Not applicable.
special instructions
ADASEL, like any other vaccine, cannot provide 100% protection for all vaccinated persons.
Since any intramuscular injection may cause hematoma at the injection site, in the presence of blood clotting disorders, for example, in hemophilia or thrombocytopenia, as well as in patients using anticoagulants, ADASEL should not be vaccinated, unless the benefits outweigh the risks. If a decision is made on intramuscular administration of the vaccine to the indicated group of persons, the drug should be used with extreme caution, in compliance with measures aimed at preventing the formation of hematoma after injection.
The development of hypersensitivity reactions to any of the components of the vaccine is possible after administration of ADASEL even in persons without a history of hypersensitivity reactions to the components of the drug.
Against the background of reduced immunity (for example, due to immunosuppressive therapy), the immune response after vaccination may not form. For this reason, if possible, vaccination should not be carried out until the end of the use of immunosuppressive drugs. This recommendation does not apply to patients with chronic immunodeficiency disorders, in particular those with HIV infection.
For progressive / unstable neurological disorders, uncontrolled epilepsy, or progressive encephalopathy, vaccination is not given until 3 conditions are met:
- The treatment regimen for these diseases has been determined.
- The state has stabilized.
- The benefits of ADASEL administration clearly outweigh the risks.
In cases where Guillain-Barré syndrome develops within 6 weeks after administration of a vaccine containing tetanus toxoid, the decision to use ADASEL or another vaccine containing tetanus toxoid should be made on the basis of a careful assessment of the ratio of the expected benefit and possible risk.
Influence on the ability to drive vehicles and complex mechanisms
The effect of ADASEL on the ability to drive vehicles has not been studied.
Application during pregnancy and lactation
ADASEL during pregnancy / lactation can only be used if the expected benefit outweighs the potential risk.
The reproductive toxicity of the drug, as well as its effect on the development of the embryo and fetus, has not been studied.
Vaccination is not recommended during pregnancy unless there is an obvious risk of contracting whooping cough. The vaccine is inactivated, so the risk to the embryo / fetus is unlikely. The physician should carefully assess the balance of benefits with the risk of using ADASEL on an individual basis in the event of an outbreak of infection in the community or in the presence of a high probability of infection from a sick family member.
The effect of the drug during lactation has not been studied. The vaccine is inactivated, so the risk of adverse effects on the mother and baby is unlikely. However, the question of the possibility / necessity of administering ADASEL is decided only individually by the doctor.
Pediatric use
For patients under 4 years of age, ADASEL is contraindicated.
Use in the elderly
This revaccination is contraindicated for persons over 64 years old.
Drug interactions
Simultaneous use of ADASEL with trivalent inactivated influenza vaccine and hepatitis B vaccine is possible.
The results in terms of immunogenicity and safety in adults are comparable both with the simultaneous administration of ADASEL and the trivalent inactivated influenza vaccine, and when these vaccines are given one month apart.
In adolescents, the immunogenicity and safety of ADASEL and the vaccine for the prevention of hepatitis B administered simultaneously and with an interval of 1 month were compared. In both cases, during the formation of the immune response, no mutual influence on any of the antigens was observed.
With the simultaneous use of ADASEL with other vaccines, each vaccine should be injected with different syringes in different parts of the body, preferably in different limbs.
The formation of an immune response can be influenced by immunosuppressive agents used simultaneously with ADASEL. Immunosuppressive therapy, including radiation therapy, the use of alkylating drugs, antimetabolites, cytotoxic drugs and glucocorticosteroids (in doses exceeding therapeutic ones), can lead to a decrease in the immune response to the vaccine.
It is prohibited to mix ADASEL in one syringe with any preparations intended for parenteral administration.
Analogs
ADASEL analogs are Adsorbed pertussis-diphtheria-tetanus vaccine (DTP-vaccine), Infanrix [Vaccine for the prevention of diphtheria, tetanus, pertussis (acellular) three-component adsorbed liquid].
Terms and conditions of storage
Store at 2-8 ° C, do not freeze. Suspension that has been frozen must not be used. Keep out of the reach of children.
The shelf life is 3 years.
Terms of dispensing from pharmacies
Dispensed by prescription.
Reviews about ADASEL
Reviews of ADASEL are few, since the drug was recently registered. Most of the responses are related to the expectation of the vaccine on the market, since it allows pertussis prophylaxis not only in children from 4 years of age, but also in adult patients.
Price for ADASEL in pharmacies
The approximate price for ADASEL (1 bottle) is 2050-2602 rubles.
ADASEL: prices in online pharmacies
|
Drug name Price Pharmacy |
|
Adacel Vaccine for the prevention of diphtheria 0.5 ml / dose suspension for intramuscular administration of 0.5 ml 1 pc. 1990 RUB Buy |

Anna Kozlova Medical journalist About the author
Education: Rostov State Medical University, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!