- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Bioparox
Bioparox: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. Drug interactions
- 12. Analogs
- 13. Terms and conditions of storage
- 14. Terms of dispensing from pharmacies
- 15. Reviews
- 16. Price in pharmacies
Latin name: Bioparox
ATX code: R02AB03
Active ingredient: fusafungin (fusafunginum)
Producer: EGIS Pharmaceutical Plant, CJSC (EGIS Pharmaceuticals PLC) (Hungary)
Description and photo updated: 2018-26-11

Bioparox is a drug for topical use in otorhinolaryngology with antibacterial and anti-inflammatory effects.
Release form and composition
Bioparox is produced in the form of an aerosol for inhalation metered: a yellow solution with a characteristic odor [10 ml each (400 inhalations) in aluminum aerosol cans with a dosing valve, in blisters 1 can, complete with spray nozzles (yellow for the nose, white - for the mouth) and an activator cap, in a cardboard box 1 pack with a case for portable carrying].
1 bottle and 1 release (squeeze) contains:
- active substance: fusafungin - 50 mg and 0.125 mg, respectively;
- auxiliary components: anhydrous ethanol, saccharin, propellant - norflurane 1,1,1,2-tetrafluoroethane (HFA-134a), isopropyl myristate, aromatic additive 14868 [ethanol 96%, anise alcohol, geranyl acetate, geraniol, isoamyl acetate, phenylethanol, methyl anthranilate, extract Carvi (cumin extract), China mint extract (field mint extract), Badian extract (anise oil), Cloves extract (clove bud extract), Coriander extract (coriander seed extract), tarragon herb wormwood oil, Rosemary extract (extract rosemary flowers), Florida Valencia orange extract (sweet orange peel extract), Peppercorn extract (pimento or paprika fruit extract), Paraguay small grain extract (orange extract), vanilla resinoid, propylene glycol, indole, heliotropin, linalistol, isopropylamine, isopropylterpineol, lignin vanillin, ethyl vanillin].
Pharmacological properties
Pharmacodynamics
Bioparox is a topical antibacterial drug with anti-inflammatory properties.
Under in vitro conditions, fusafungin is active against the following microorganisms: pneumococci (Pneumococci), group A streptococci (group A Streptococci), staphylococci (Staphylococcus), certain strains of Neisseria (Neisseria), fungi of the genus Candida (Candida albicans), some mycoplasma bacteria (Mycoplasma pneumoniae). A similar effect of the drug in vivo is expected.
The pronounced anti-inflammatory effect of fusafungin is due to a decrease in the concentration of tumor necrosis factor (TNF-alpha) and suppression of the synthesis of free radicals by macrophages while maintaining phagocytosis.
Pharmacokinetics
After spraying Bioparox, fusafungin is distributed mainly in the oropharynx and nasal cavity. In blood plasma, it is found in a very low concentration (no more than 1 ng / ml) and has no systemic effect.
The results of long-term laboratory studies on animals confirm the absence of teratogenic effects on the fetus, genotoxic and embryotoxic effects.
Indications for use
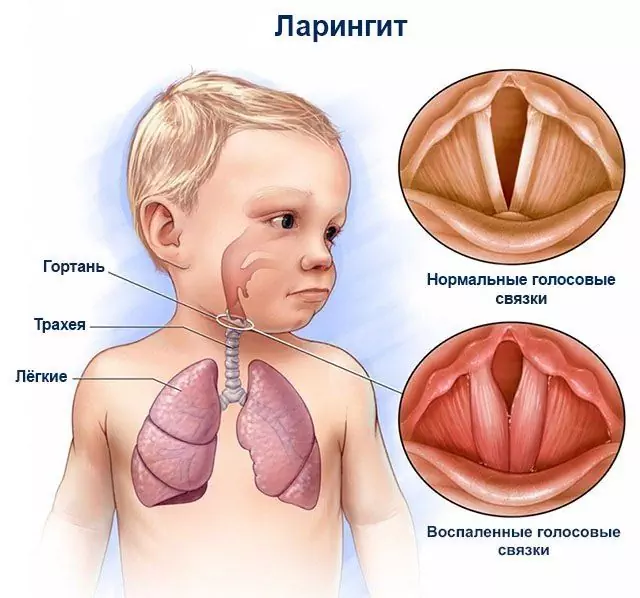
According to the instructions, Bioparox is indicated for the treatment of the following inflammatory diseases of the respiratory tract of infectious etiology: pharyngitis, rhinitis, rhinopharyngitis, sinusitis, laryngitis, tonsillitis, conditions after tonsillectomy.
Contraindications
- predisposition to allergic reactions or bronchospasm;
- breast-feeding;
- hypersensitivity to the components of the drug.
Bioparox is not prescribed for children under 12 years of age.
The drug should be prescribed with caution during pregnancy.
Instructions for the use of Bioparox: method and dosage
Bioparox is applied topically, by inhalation release into the oral cavity and / or into the nasal passage.
After opening the package, the cylinder should be activated by pressing 4 times on its base.
The set includes special nozzles for inhalation through the mouth or nose. The procedure should be carried out by holding the balloon in an upright position between the thumb and forefinger with the nozzle facing up.
When treating rhinitis, rhinopharyngitis, sinusitis, the drug is injected through the nose using a yellow nozzle. The nose should be thoroughly cleaned before the procedure. Having fastened the nozzle to the balloon, it is inserted into one of the nasal passages, while holding the opposite nasal passage with the finger of the free hand. In the vertical position of the balloon, it is necessary to make 2 energetic presses against the stop on its base, closing your mouth and holding your breath.
When treating pharyngitis, tonsillitis, laryngitis or conditions after tonsil removal, Bioparox is administered through the mouth using a white nozzle. While holding the balloon upright, the white nozzle is inserted into the mouth and tightly wrapped around it with the lips. Holding your breath, you should make 4 energetic clicks all the way to the base of the balloon.
Disinfection of the nozzles for the mouth and nose is carried out every other day using a cotton swab dipped in 90% ethyl alcohol.
The can should be placed in a portable carrying case and carried with you at all times.
Recommended dosage for patients over 12 years old: 4 releases (inhalations) into the oral cavity and / or 2 releases into each nasal passage 4 times a day. The duration of the course of treatment is no more than 7 days.
The maximum therapeutic activity of Bioparox is achieved with strict adherence to the prescribed dosage regimen. Do not stop using the aerosol if symptoms of improvement appear. Interruption of the course of therapy can cause a relapse of the disease.
If, against the background of the use of Bioparox, symptoms of the disease and / or an increased body temperature persist, you should consult your doctor. With pronounced clinical manifestations of bacterial infection, additional administration of systemic antibiotics may be required.
Side effects
- from the respiratory system: very often - sneezing; often - dry throat and / or nose, cough, throat irritation; very rarely - shortness of breath, asthmatic attacks, bronchospasm, laryngospasm, Quincke's edema (including laryngeal edema);
- allergic reactions: very rarely - transient local reactions, usually with an individual predisposition to allergies;
- from the immune system: very rarely - anaphylactic shock;
- dermatological reactions: very rarely - itching, rash, urticaria;
- general disorders and symptoms: very often - unpleasant taste in the mouth, redness of the mucous membrane of the eyes; often - nausea, dryness of the mucous membranes of the respiratory tract, cough, sensation of irritation in the throat; frequency not established - vomiting.
Overdose
Symptoms of an overdose of Bioparox are dizziness, a feeling of numbness in the mouth against the background of circulatory disturbances, burning sensation and increased pain in the throat.
The appointment of symptomatic therapy, careful monitoring of the patient's condition is recommended.
special instructions
Since Bioparox is an antibacterial drug, in order to avoid the development of superinfection, the duration of the recommended course of therapy (more than 7 days) must not be exceeded.
The therapeutic effect is assessed by the doctor after the end of the course of treatment.
The development of general disorders usually does not require discontinuation of treatment. If symptoms of allergic reactions appear, the aerosol spraying should be stopped and the patient is advised to consult a doctor. Due to the increased risk of anaphylactic shock with pruritus, generalized erythema, respiratory or laryngeal symptoms, immediate intramuscular administration of epinephrine (adrenaline) is indicated. The dose is determined at the rate of 0.01 mg per 1 kg of patient weight. If necessary, after 20 minutes, the injection should be repeated at the same dose.
Aerosol exposure due to the presence of propylene glycol may irritate the skin.
Do not spray the drug into the eyes.
The ethanol content in one dose is less than 100 mg.
The cylinder should be stored in a case to prevent damage during transport. It should not be thrown into fire, even if it is not inside the drug.
Influence on the ability to drive vehicles and complex mechanisms
Bioparox does not affect the speed of psychomotor reactions and the patient's ability to drive vehicles.
Application during pregnancy and lactation
The safety and efficacy of the drug during pregnancy has not been confirmed by the results of clinical studies. Care should be taken when prescribing Bioparox during gestation.
The use of the drug during breastfeeding is contraindicated.
Pediatric use
Bioparox is contraindicated in children under 12 years of age.
Drug interactions
Drug interaction with topical application of fusafungin with other drugs has not been established.
Analogs
Bioparox analogs are: Gramicidin C, Grammidin with anesthetic neo, Trakhisan, Gramicidin paste, Grammidin, Isofra.
Terms and conditions of storage
Keep out of the reach of children.
Store at room temperature, away from sources of strong heat, do not expose to heat above 50 ° C.
Shelf life is 2 years.
Terms of dispensing from pharmacies
Available without a prescription.
Reviews about Bioparox
Reviews about Bioparox are mostly positive. Patients point to its fairly rapid action and good efficacy in the treatment of infectious and inflammatory diseases of the respiratory system. The advantages of the drug include its local antibacterial effect and ease of use.
The disadvantage of the spray is a burning sensation in the throat, a specific taste, a pungent odor.
Price for Bioparox in pharmacies
The price for Bioparox for 1 cylinder can be from 497 rubles.

Anna Kozlova Medical journalist About the author
Education: Rostov State Medical University, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!