- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Humalog Mix 50
Humalog Mix 50: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. In case of impaired renal function
- 12. For violations of liver function
- 13. Drug interactions
- 14. Analogs
- 15. Terms and conditions of storage
- 16. Terms of dispensing from pharmacies
- 17. Reviews
- 18. Price in pharmacies
Latin name: Humalog Mix 50
ATX code: A10AD04
Active ingredient: insulin lispro (insulin lispro)
Producer: LILLY FRANCE (France)
Description and photo update: 2018-27-11
Prices in pharmacies: from 1681 rubles.
Buy

Humalog Mix 50 is a hypoglycemic drug containing a combination of short and medium acting insulin analogs.
Release form and composition
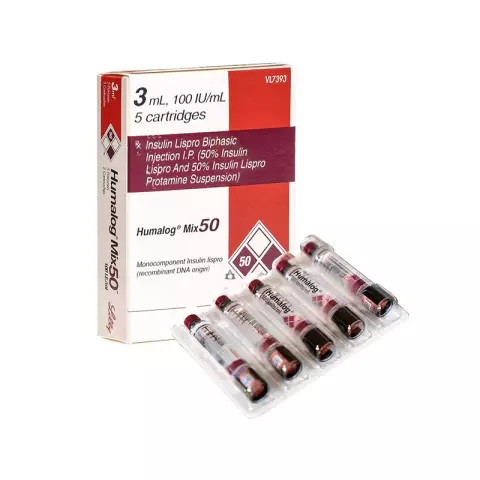
Dosage form - suspension for subcutaneous administration: white, exfoliating with the formation of a precipitate and a transparent almost colorless or colorless supernatant liquid, quickly resuspended with gentle shaking (3 ml in a cartridge, 5 cartridges in a blister, in a cardboard box 1 blister; 3 ml in a cartridge, built into the QuickPen syringe pen, in a cardboard box 5 syringe pens; each pack also contains instructions for the use of Humalog Mix 50).
Composition of 1 ml of suspension:
- active substance: insulin lispro - 100 International units (ME);
- auxiliary components: sodium hydrogen phosphate heptahydrate, protamine sulfate, liquid phenol, glycerol (glycerin), metacresol, zinc oxide, 10% sodium hydroxide solution and / or 10% hydrochloric acid solution, water for injection.
Pharmacological properties
Pharmacodynamics
Humalog Mix 50 is a ready-made mixture containing 50% solution of insulin lispro (a fast-acting analogue of human insulin) and 50% protamine suspension of insulin lispro (an analogue of human insulin of medium duration).
The main property of the drug is the regulation of glucose metabolism. It also has anti-catabolic and anabolic effects on various body tissues. In muscle tissue, under the influence of Humalog Mix 50, the content of fatty acids, glycerol and glycogen increases, protein synthesis increases, and the consumption of amino acids increases. This reduces glycogenolysis, gluconeogenesis, lipolysis, ketogenesis, protein catabolism and amino acid release.
Insulin lispro has been found to have a molarity equivalent to human insulin, but its effect develops faster and lasts less.
After the introduction under the skin, there is a rapid onset of action of insulin lispro and an early onset of its peak. Humalog Mix 50 begins to work approximately 15 minutes after injection, so it can be administered right before meals (0-15 minutes), unlike regular human insulin.
The action profile of insulin lispro protamine is similar to that of regular insulin isophane with a duration of action of about 15 hours.
Pharmacokinetics
The pharmacokinetics of Humalog Mix 50 is determined by the individual pharmacokinetic properties of its two active components.
The degree of absorption and the onset of action of the drug depend on the place of administration of the suspension (thigh, abdomen, buttocks) and its dose, as well as on the patient's physical activity, body temperature and blood supply.
Insulin lispro is rapidly absorbed after subcutaneous administration. The maximum concentration in the blood reaches after 30-70 minutes.
The pharmacokinetic parameters of insulin lispro protamine are similar to those of insulin isophane (insulin of average duration of action).
In renal and hepatic insufficiency, insulin lispro is absorbed more rapidly than soluble human insulin.
Indications for use
Humalog Mix 50 is used for diabetes mellitus requiring insulin therapy behavior.
Contraindications
Absolute:
- hypoglycemia;
- age up to 18 years;
- hypersensitivity to any component of Humalog Mix 50.
Relative:
- renal / hepatic impairment;
- emotional stress, increased physical activity or a change in the usual diet (insulin dose adjustment may be required);
- long-term course of diabetes mellitus, diabetic neuropathy or the simultaneous use of beta-blockers (a change or decrease in the severity of symptoms-precursors of hypoglycemia is possible);
- pregnancy and the period of breastfeeding.
Humalog Mix 50, instructions for use: method and dosage
Humalog Mix 50 is intended for subcutaneous administration only. You can enter it immediately before meals or after meals. The dose is determined by the doctor individually for each patient, based on the level of glucose in the blood.
The drug can be injected into the abdomen, thigh, shoulder or buttocks. The injection sites should be alternated in such a way that the suspension is, if possible, injected into the same place no more than once a month.
When administering Humalog Mix 50, care must be taken to prevent the suspension from getting into the lumen of blood vessels. No need to massage the injection site after injection.
Application of the drug in cartridges
The rules for installing the cartridge into the device for administering the drug and recommendations for attaching the needle to it before insertion are described in the manufacturer's instructions for the device intended for insulin administration. It is important to follow the directions strictly.
Warm up to room temperature before administration. Immediately before the injection, the cartridge must be rolled 10 times between the palms and shaken 10 times, turning it 180 °, so that the insulin resuspended, i.e., acquired the appearance of a homogeneous turbid liquid. It is not necessary to shake the cartridge vigorously, as this may result in foam formation, which makes it difficult to correctly set the dose. A small glass ball is provided inside the cartridge to facilitate mixing of the drug.
If after mixing the suspension does not acquire a homogeneous consistency (flakes are visible), it must not be used!
Dosage rules for Humaloga Mix 50:
- Wash the hands.
- Choose an injection site and prepare the skin according to your doctor's recommendations.
- Remove the outer protective cap from the needle.
- Fix the skin by gathering it in a small fold.
- Insert the needle under the skin into the assembled fold and inject, following the directions for using a pen.
- Remove the needle and gently press the insertion site with a cotton swab for a few seconds. Do not rub the injection area.
- Unscrew the needle using the outer protective cap and discard.
- Put the cap on the pen syringe.
Application of Humaloga Mix 50 in a QuickPen syringe pen
The QuickPen syringe pen is a special device designed to deliver insulin (the so-called insulin pen syringe). It contains 3 ml of the drug (300 IU), allows for one injection to inject from 1 to 60 units of insulin, while the dose can be set with an accuracy of one unit.
The blue color of the QuickPen syringe barrel indicates that it is intended for use with Humalog products. The color of the injection button on the pen corresponds to the color of the strip on the pen label and depends on the type of insulin.
The QuickPen pen is recommended for use with appropriate needles from Becton, Dickinson and Company (BD).
Each pen is designed for individual use. It should not be passed on to others, as this carries the risk of contracting infectious diseases. For each injection, a new needle must be used and before insertion, make sure that it is fully attached to the syringe pen.
It is forbidden to use a syringe pen if any of its parts is broken or damaged. Patients are advised to always carry a spare syringe in case of loss or breakage.
Humalog Mix 50 in the QuickPen pen is not recommended for use by patients with impaired vision.
Recommendations for preparing for injection:
- Carefully follow the rules of antiseptics and asepsis recommended by the attending physician.
- Wash the hands.
- Choose an injection site, wipe the skin.
Instructions for the preparation of the QuickPen syringe pen and the introduction of Humalog Mix 50:
- Pulling to remove the pen cap. Do not rotate the cap, do not remove the label from the syringe. Make sure that the correct type of insulin is selected and that it is up to date. Check the appearance of the suspension.
- Get a new needle. Remove the paper sticker from the outer cap. Wipe the rubber disc at the end of the cartridge holder with a cotton swab moistened with alcohol. Put the needle in the cap on the syringe-pen straight along the axis and screw it until it is completely attached.
- Remove the outer cap from the needle (do not discard). Then remove the inner cap (you can throw it away).
- Check the pen syringe for insulin intake (drug trickle appears). This should be done every time before injection to make sure the pen is ready to deliver the required dose, otherwise you can enter too little or too much dose.
- Fix the skin by pulling and gathering it into a large fold. Insert the needle under the skin as directed by your doctor. Rotate the dose button to the required number of insulin units. With your thumb in a straight axis, firmly press the button until it stops. To fully inject the dose, hold down the button and slowly count to 5.
- Remove the needle and gently press the injection site with a cotton swab for a few seconds without rubbing it. The presence of a drop of the drug on the tip of the needle is normal and does not affect the dose. If the suspension is dripping from the needle, it is most likely that the patient did not hold the needle under the skin for a sufficient amount of time to completely inject the drug.
- Place the outer cap on the needle. Remove it from the pen to prevent air bubbles from entering the cartridge.
Even numbers in the indicator window are printed as numbers, odd numbers as straight lines between even numbers.
If you need to inject a dose that exceeds the number of units of insulin remaining in the cartridge, you can inject the remaining drug and then use a new pen or use a new pen immediately.
Do not try to change your insulin dose while injecting.
Important information! The pen does not allow you to set a dose in excess of the number of units that remain in the cartridge. In the event that the patient is not sure whether he has administered the full dose, another should not be administered.
Features of storage and disposal of syringe pens:
- do not use a syringe pen if it has been stored outside the refrigerator for more than the time specified in the instructions;
- do not store the syringe pen with the attached needle (the drug may leak out or dry out inside the needle, causing it to become clogged, and air bubbles may also form inside the cartridge);
- Unused pen pens should be refrigerated at 2-8 ° C. Do not use the drug if it has been frozen;
- the pen used in the current period must be stored at room temperature (no more than 30 ° C), away from sunlight and heat sources;
- Used needles should be disposed of in sealed, puncture-proof containers;
- the filled needle container must not be recycled;
- Used syringe pens (without needles) should be disposed of in accordance with the recommendations of your doctor and in accordance with local regulations for the disposal of medical waste.
Side effects
The most common side effect seen with all types of insulin is hypoglycemia. In severe cases, it can cause loss of consciousness, in exceptional cases - lead to death.
Sometimes local allergic reactions occur: redness, itching or swelling at the injection site. As a rule, these phenomena disappear on their own within a few days / weeks. In some patients, they are not associated with the use of insulin, but are caused, for example, by inappropriate administration of the drug or skin irritation after using a cleansing agent.
Insulin rarely causes systemic allergic reactions, but they are more serious. May be manifested by the following symptoms: shortness of breath, difficulty breathing, decreased blood pressure, tachycardia, increased sweating, generalized itching. In the event of a severe allergic reaction, urgent treatment is required. These patients may require desensitizing therapy or insulin changes.
With prolonged treatment, lipodystrophy may develop at the injection site.
There are isolated cases of edema, mainly with rapid normalization of blood glucose levels against the background of intensive insulin therapy with initially unsatisfactory glycemic control.
Overdose
With an overdose of insulin, hypoglycemia develops, accompanied by pallor of the skin, increased sweating, lethargy, headache, confusion, tremors, tachycardia, vomiting. Under some conditions (for example, in the case of intensive control of diabetes mellitus or with a long duration of diabetes mellitus), the symptoms of hypoglycemia may change.
Hypoglycemia in most cases is stopped by ingestion of sugar or glucose. As a treatment, correction of insulin, diet and / or physical activity is carried out.
Hypoglycemia of moderate severity is corrected by intramuscular or subcutaneous administration of glucagon, then the patient is recommended to take oral carbohydrates.
Severe hypoglycemia can lead to neurological disorders, seizures, and coma. Such patients are prescribed intramuscular or subcutaneous administration of glucagon or intravenous administration of a concentrated solution of glucose (dextrose). In order to prevent the recurrence of hypoglycemia, after the restoration of consciousness, the patient needs to eat food rich in carbohydrates. The patient must be under the supervision of a physician.
special instructions
Careful medical supervision is required when a patient is switched to a different type of insulin or insulin with a different trade name. When changing the brand (manufacturer), species (animal insulin, human or human analog), type (soluble insulin, insulin isophane, etc.) and / or production method of the drug (DNA recombinant insulin or animal insulin), correction may be required dose.
When transferring a patient from insulin of animal origin to human insulin, a dose adjustment may be required, and even at the first administration of the drug, or gradually over several weeks / months of therapy.
Hypo- and hyperglycemic states must be corrected, otherwise they can lead to loss of consciousness, coma and even death. It should be borne in mind that the symptoms-precursors of hypoglycemia may change, their severity may decrease with a prolonged course of diabetes mellitus or diabetic neuropathy, as well as with the simultaneous use of beta-blockers.
The introduction of inadequate doses and the cancellation of Humalog Mix 50, especially in type 1 diabetes mellitus, can cause hyperglycemia and diabetic ketoacidosis - conditions that pose a potential threat to the patient's life.
With some diseases and emotional stress, the need for insulin may increase.
Dose adjustment of Humaloga Mix 50 may be required in case of changes in the usual diet or increased physical activity. Increased physical activity sometimes increases the risk of hypoglycemia.
The cartridges with the drug must be used with syringe pens bearing the CE marking.
To prevent transmission of a possible infectious disease, each cartridge or pen should only be used by one patient, even after changing the needle.
Influence on the ability to drive vehicles and complex mechanisms
With the development of hypoglycemia, a decrease in the speed of reactions and concentration of attention is possible, which increases the risk of injury when performing potentially dangerous activities, including driving a car and working with complex mechanisms. In this regard, caution should be exercised, especially in patients in whom symptoms of hypoglycemia are absent or mild. In the case of frequent development of hypoglycemia, the feasibility of performing activities with possible dangerous consequences should be assessed.
Application during pregnancy and lactation
There have been no adequate and well-controlled studies in pregnant women. In the course of experiments on animals, there were no violations of fertility and a negative effect of the drug on the fetus. However, it is known that the effects obtained as a result of studies of the effect of drugs on reproduction in animals are not always comparable with those when the drug is exposed to the human body. In this regard, during pregnancy, Humalog Mix 50 can be used only in case of a clinically justified need.
If pregnancy occurs during therapy, you should warn your doctor, since during this period it is especially important to monitor the condition and the treatment process. In the first trimester, the need for insulin usually decreases, in the II and III trimesters, it increases. During and immediately after childbirth, the need for insulin can decrease dramatically.
During breastfeeding, women with diabetes may need to adjust their insulin dose and / or diet.
Pediatric use
Humalog Mix 50 is not used to treat children and adolescents under the age of 18, since there is no information on the efficacy and safety of the drug in patients of this age category.
With impaired renal function
In case of renal failure, Humalog Mix 50 should be used with caution, under the close supervision of a physician, since the need for insulin may decrease.
For violations of liver function
In case of liver failure, Humalog Mix 50 should be used with caution, under the close supervision of a physician, since the need for insulin may decrease due to a decrease in the ability to gluconeogenesis and a decrease in insulin metabolism. However, in chronic liver failure, increased insulin resistance is possible, which requires an increase in dose.
Drug interactions
The hypoglycemic effect of Humalog Mix 50 is reduced by beta 2 -adrenomimetics (for example, terbutaline, salbutamol, ritodrin), glucocorticosteroids, phenothiazine derivatives, thiazide diuretics, iodine-containing thyroid hormones, oral contraceptives.
The hypoglycemic effect of Humalog Mix 50 is enhanced by oral hypoglycemic drugs, sulfanilamide antibiotics, anabolic steroids, beta-blockers, angiotensin-converting enzyme inhibitors (captopril, enapril), antagonists of angiotensin II receptors, some antidepressants, acetalicylic acid inhibitors (e.g., ethanol and ethanol-containing drugs, octreotide, guanethidine, fenfluramine.
With the simultaneous use of drugs of the thiazolidinedione group, an increase in the risk of edema and heart failure is possible, especially in patients with diseases of the cardiovascular system and the presence of risk factors for chronic heart failure.
Reserpine, clonidine and beta-blockers can mask the symptoms of hypoglycemia that developed during the use of Humalog Mix 50.
The interaction of Humalog Mix 50 with other insulin preparations has not been studied.
The possibility of using any other drugs during the treatment of diabetes mellitus should be agreed with the attending physician.
Analogs
Analogues of Humaloga Mix 50 are: NovoMix 30 Penfill, NovoMix 30 FlexPen, NovoMix 50 FlexPen, NovoMix 70 FlexPen, Insulin Aspart, NovoRapid Penfill, NovoRapid FlexPen, Lantus SoloStar, Tudgeo SoloStar, InsuindetlinPen Homorap 40, etc.
Terms and conditions of storage
Keep out of reach of children, protected from direct sunlight and sources of heat, at a temperature of 2-8 ° C. Do not freeze. The drug in use can be stored at temperatures up to 30 ° C, but not more than 28 days.
The shelf life is 3 years.
Terms of dispensing from pharmacies
Dispensed by prescription.
Reviews of Humalog Mix 50
According to reviews, Humalog Mix 50 is a drug that effectively lowers blood sugar levels, is well tolerated and does not cause side effects if all the doctor's recommendations are followed. Especially patients note the convenience of using the cartridges built into the QuickPen syringe pens.
Price for Humalog Mix 50 in pharmacies
The approximate price for Humalog Mix 50 is 1767-1998 rubles. for 5 KvikPen syringe pens of 3 ml.
Humalog Mix 50: prices in online pharmacies
|
Drug name Price Pharmacy |
|
Humalog Mix 50 100 IU / ml suspension for subcutaneous administration 3 ml 5 pcs. 1681 RUB Buy |

Maria Kulkes Medical journalist About the author
Education: First Moscow State Medical University named after I. M. Sechenov, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!