- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Kutiveit
Kutiveit: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. In case of impaired renal function
- 12. For violations of liver function
- 13. Use in the elderly
- 14. Drug interactions
- 15. Analogs
- 16. Terms and conditions of storage
- 17. Terms of dispensing from pharmacies
- 18. Reviews
- 19. Price in pharmacies
Latin name: Cutivate
ATX code: D07AC17
Active ingredient: fluticasone (fluticasone)
Manufacturer: GlaxoSmithKline Pharmaceuticals (Poland)
Description and photo update: 2019-26-08

Kutiveit is an external glucocorticosteroid drug used in the treatment of dermatoses.
Release form and composition
Kutiveit is available in the following dosage forms:
- Ointment for external use: translucent, homogeneous, almost white or white (15 g each in aluminum tubes, 1 tube each in a cardboard box);
- Cream for external use: soft, homogeneous, almost white or white (15 g each in aluminum tubes, 1 tube each in a cardboard box).
The composition of 1000 mg of ointment includes:
- Active ingredient: fluticasone propionate - 0.05 mg (0.005%);
- Auxiliary components: microcrystalline wax - 25%, propylene glycol - 5%, sorbitan sesquioleate - 0.5%, liquid paraffin - up to 100%.
The 1000 mg cream contains:
- Active ingredient: fluticasone propionate - 0.5 mg (0.05%);
- Auxiliary components: isopropyl myristate - 5%, liquid paraffin - 40%, ketomacrogol 1000 - 0.75%, cetostearyl alcohol - 5.25%, propylene glycol - 10%, sodium phosphate - 0.15%, imidourea - 0.2%, citric acid monohydrate - 0.05%, purified water - up to 100%.
Pharmacological properties
Pharmacodynamics
The active ingredient of Kutiveyta is fluticasone - a glucocorticosteroid (GCS) for external use, which has an anti-inflammatory, antipruritic and vasoconstrictor effect.
The anti-inflammatory property is due to numerous mechanisms of inhibition of the late phase of allergic reactions: fluticasone reduces the number of mast cells, weakens chemotaxis and activation of eosinophils, inhibits the metabolism of arachidonic acid, and also reduces the production of cytokines by monocytes, lymphocytes, eosinophils and mast cells.
When Kutiveyta is used externally, the likelihood of suppression of the function of the hypothalamic-pituitary-adrenal system (HPA) is insignificant. In vitro studies have shown that fluticasone has a high selectivity and affinity for glucocorticoid receptors. According to the data of clinical studies, when fluticasone enters the systemic circulation, it is rapidly transformed in the liver to an inactive metabolite (carboxylic acid) and is rapidly excreted from the body. Due to these properties, Kutiveit is characterized by a high therapeutic index.
Fluticasone does not cause unexpected hormonal disturbances, does not significantly affect the gastrointestinal tract, respiratory, central and peripheral nervous, cardiovascular systems.
Pharmacokinetics
When applied externally, a limited amount of fluticasone is absorbed through the skin, so its bioavailability is very low. When taken orally, the bioavailability of fluticasone is close to zero due to low absorption from the gastrointestinal tract and extensive metabolism during the first passage through the liver. Due to this, if the drug is accidentally swallowed, the systemic exposure is low.
When absorbed into the systemic circulation, fluticasone quickly enters the bile and is excreted through the intestines. The drug does not bind to melanin, does not accumulate in tissues.
In preclinical and clinical studies, it has been established that fluticasone has a high metabolic clearance and is rapidly excreted from the body. Thus, the drug entering the systemic circulation through the skin is rapidly inactivated. Fluticasone is metabolized by hydrolysis to carboxylic acid, a metabolite with very weak anti-inflammatory and glucocorticoid activity.
Fluticasone is excreted mainly through the intestines within 48 hours.
Indications for use
According to the instructions, Kutiveit is prescribed for the treatment of dermatoses (acute and chronic):
- Eczema;
- Discoid lupus erythematosus;
- Dermatitis (atopic, contact, seborrheic);
- Lichen planus;
- Neurodermatosis, including lichen simplex;
- Psoriasis;
- Erythroderma (as an adjunct to systemic glucocorticosteroid therapy);
- Insect bites.
Also, the drug is used to reduce the risk of recurrence of atopic eczema, occurring in a chronic form, in cases of a therapeutic effect in the treatment of the acute phase of the disease.
Contraindications
- Acne rosacea;
- Perioral dermatitis;
- Acne vulgaris;
- Fungal and viral skin infections;
- Genital and perianal itching;
- Age up to 6 months (for ointment), up to 1 year (for cream);
- Hypersensitivity to drug components.
Nursing and pregnant women should use Kutiveit with caution.
Instructions for the use of Kutiveyta: method and dosage
Kutiveit ointment should be used to treat dry, flaky and lichenified lesions, Kutiveit cream - for wet and weeping lesions.
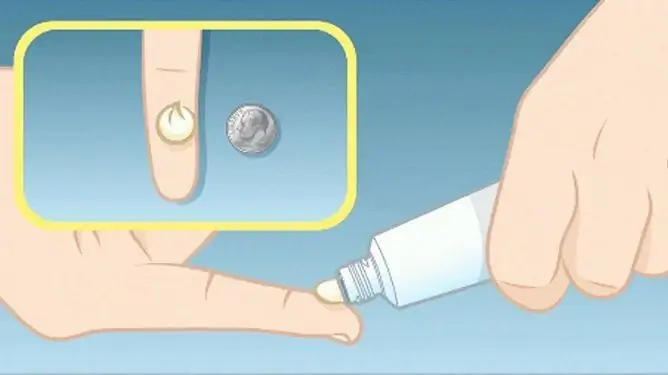
In the treatment of acute and chronic dermatoses, the agent should be applied 1-2 times a day in a thin layer in an amount not exceeding that required to cover the entire affected area, and rub in gently. The course of therapy is until the effect is achieved, but not more than 4 weeks. In the future, the frequency of use of the drug is reduced or Kutiveit is replaced with a less active glucocorticosteroid.
After applying the ointment / cream, allow sufficient time for the preparation to be absorbed before using the emollient.
If within 2 weeks of treatment the condition does not improve or worsens, you should consult a doctor to re-evaluate the therapy and clarify the diagnosis.
Cancellation Kutiveit should be after achieving control of the disease, gradually, against the background of maintenance therapy with an emollient. With a sharp cancellation of GCS, it is possible to resume the initial symptoms of the disease.
In the treatment of atopic dermatitis, to reduce the risk of relapse after achieving a therapeutic effect in the acute phase of the disease, the frequency of application of the ointment / cream is reduced to 2 times a week, without the use of occlusive dressings. The drug should be applied to all areas of the body that were previously affected or where a relapse can be expected. This dosing regimen of Kutiveite must be combined with the daily use of emollients, and constant monitoring of the patient's condition is also required.
Side effects
When using Kutiveyta, the following side effects may occur:
- Local reactions: itching and burning at the site of application of the drug (with the development of symptoms of a hypersensitivity reaction, therapy should be stopped immediately), atrophic skin changes (hypertrichosis, striae, thinning of the skin, dilation of superficial blood vessels, hypopigmentation), exacerbation of signs of dermatosis, development of allergic contact dermatitis; sometimes - the occurrence of secondary infections (especially when using occlusive dressings and when applying Kutiveite to the area of skin folds);
- Systemic reactions: with prolonged use in high doses or when Kutiveyta is applied to large areas of the skin, significant systemic absorption of the drug is possible with the development of symptomatic hypercortisolism. Most often this is observed in young and young children, as well as when using occlusive dressings (in children, diapers and diapers can perform their function). In rare cases, the use or withdrawal of glucocorticosteroids in the treatment of psoriasis can provoke the onset of a pustular form of the disease.
Overdose
Despite external use, fluticasone can be absorbed through the skin in an amount sufficient for the development of systemic action. The likelihood of an acute overdose is very low, however, with chronic overdose and improper use of Kutiveite, signs of hypercortisolism may develop (Itsenko-Cushing syndrome).
If symptoms of an overdose appear, Kutiveit should be gradually canceled, reducing the frequency of its application, or replaced with less active GCS to prevent the development of glucocorticoid insufficiency. Overdose treatment is symptomatic.
special instructions
Long-term therapy at high doses over large areas of the body, especially in young children and young children, can lead to suppression of the adrenal cortex.
When applied externally in children, glucocorticosteroids can be absorbed in relatively large quantities, therefore, children are more susceptible to systemic effects of the drug than adults. In this regard, it is recommended to use Kutiveit in children in the lowest doses that provide a therapeutic effect.
When treating diseases such as discoid lupus erythematosus, psoriasis and severe eczema, it should be borne in mind that atrophic changes with prolonged external use of potent glucocorticoid drugs occur more often on the face than on other parts of the body.
When applying Kutiveite on the eyelids, avoid contact with the eyes, as this can lead to the development of glaucoma or local irritation.
During the entire course of treatment, it is recommended to regularly monitor the patient's condition.
In psoriasis, Kutiveit should be used with caution, since during therapy it is possible to develop generalized pustular psoriasis, tolerance, local or systemic toxic effects caused by a violation of the barrier function of the skin (therapy should be carried out under close monitoring of the patient's condition).
With the development of secondary infection, appropriate antibacterial drugs should be used. When signs of spreading infection appear, Kutiveit should be canceled and systemic antibacterial drugs should be prescribed.
In humid and warm conditions (including under occlusive dressings), the risk of bacterial infections increases, so the skin must be thoroughly treated with antiseptic agents each time before applying a new dressing.
The drug therapy is recommended to be combined with the use of emollient cosmetics.
Influence on the ability to drive vehicles and complex mechanisms
Special studies that would allow assessing the degree of Kutiveite's influence on cognitive and psychomotor functions of a person have not been conducted. Given the profile of adverse reactions, it is not assumed that the drug may have an adverse effect on the ability to drive vehicles and perform potentially hazardous types of work.
Application during pregnancy and lactation
There is no necessary information to assess the degree of influence of external dosage forms of GCS on human fertility.
Data on the use of fluticasone in pregnancy are limited. In preclinical studies, it was found that when used externally in pregnant animals, corticosteroids can cause deviation of embryonic development, but the significance of this phenomenon for humans has not been established. In this regard, the doctor can prescribe Kutiveit to pregnant women only if the expected benefit, according to his conclusion, is higher than the potential risks. During treatment, it is recommended to use the drug in the lowest effective dose for the shortest possible course.
The safety of fluticasone when used externally during lactation has not been established. In preclinical studies, fluticasone, administered subcutaneously in doses sufficient to determine the substance in blood plasma, was also found in mother's milk. However, there are no data confirming systemic absorption of the drug in a volume sufficient to detect the active substance in breast milk when used externally. In this regard, women who are breastfeeding are prescribed Kutiveit with caution if the benefits of therapy outweigh the possible risks. To avoid accidental swallowing of the drug by a child, it is forbidden to apply cream / ointment to the breast area.
Pediatric use
Kutiveit ointment is contraindicated under the age of 6 months, from 6 months to 12 years should be used with caution.
Kutiveit cream is contraindicated under the age of 1 year, from 1 year to 12 years should be used with caution.
Children are more likely to develop side effects of GCS, both local and systemic. In this regard, the treatment should be carried out under the supervision of a physician, the drug must be used in a minimum amount for the shortest possible period. The course of therapy should not exceed four weeks.
With impaired renal function
With prolonged use of the cream / ointment on large areas of the skin, systemic absorption of the drug is possible. In patients with impaired renal function, the metabolism and excretion of fluticasone slows down, which increases the risk of developing systemic toxicity. In this regard, patients with renal insufficiency should be used Kutiveit with caution, in the minimum effective dose for the shortest possible period, sufficient to achieve a clinical effect.
For violations of liver function
In case of impaired liver function in the case of systemic absorption of the drug (with prolonged use of the cream / ointment over large areas of the body), the metabolism and excretion of fluticasone slows down, which increases the likelihood of systemic toxicity. In this regard, with hepatic insufficiency, Kutiveit should be used with caution, in the minimum effective dose for the shortest possible period, sufficient to achieve a clinical effect.
Use in the elderly
In the course of clinical studies, differences in the results between the groups of young and elderly patients were not identified. However, in elderly patients, renal and / or hepatic function is often reduced, as a result of which it is possible to slow down the elimination of fluticasone from the body in the case of its systemic absorption. In this regard, elderly patients should use Kutiveit with caution, in the minimum effective dose for the shortest possible period of time sufficient to obtain a clinical effect.
Drug interactions
With the simultaneous use of drugs that inhibit the isoenzyme CYP3A4 (for example, itraconazole or ritonavir), inhibition of the metabolism of fluticasone is possible, as a result of which its systemic effect is enhanced. The degree of clinical significance of this interaction depends on the activity of the inhibitor of the isoenzyme CYP3A4, the method of administration of GCS and its dose, however, with external use, the likelihood of such an interaction is low.
Analogs
Kutiveit's analogs are: Nazarel, Fliksonase, Flixotide, Fluticasone, Avecort, Advantan, Akriderm, Afloderm, Beloderm, Hydrocortisone, Dermovate, Cloveit, Kutiveyt, Latikort, Lokoid, Momat, Momederm, Mometason, Predictorium, Sinodirmor Elokom and others.
Terms and conditions of storage
Keep out of reach of children at temperatures up to 30 ° C.
Shelf life is 2 years.
Terms of dispensing from pharmacies
Available without a prescription.
Reviews about Kutiveite
Reviews about Kutiveyte are mostly positive. The drug is used in various cases, with its help, various skin lesions (including on the face) are eliminated due to allergic reactions, chronic disorders and insect bites.
Most patients note the high efficiency of the ointment and cream, their quick action. According to reviews, improvement in the condition often occurs after the first application of the product. Additional advantages of Kutiveit include the possibility of treating children, good tolerance, the absence of odor in the cream / ointment and their economical consumption, the availability of the drug in pharmacies.
Opinions about the cost of the ointment and cream were divided: some consider it acceptable, others - too high.
Price for Kutiveit in pharmacies
Approximate prices Kutiveyta: cream for external use - 288-386 rubles. per tube 15 g, ointment for external use - 289-377 rubles. per tube 15 g.

Anna Kozlova Medical journalist About the author
Education: Rostov State Medical University, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!