- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Wine acid

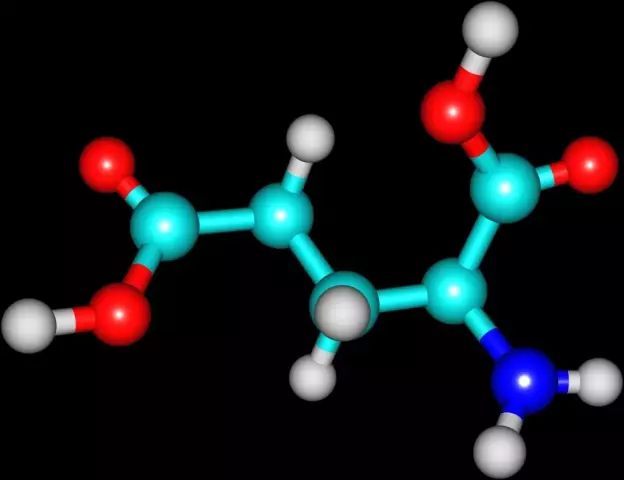
Tartaric acid is an organic compound - a dibasic hydroxy acid with the formula HOOC-CH (OH) -CH (OH) -COOH.
Tartaric acid (otherwise dioxysuccinic or tartaric acid) is odorless and colorless crystals that have a very sour taste.
As a food additive, tartaric acid is named E334.
Tartaric acid is found naturally in many fruits. It is especially abundant in grapes and citrus fruits. In some foods, it is combined with magnesium, calcium or potassium.
Initially, tartaric acid was obtained as a by-product of the wine industry. It was mainly used to prevent the growth of bacteria in wine vats and barrels.
Getting tartaric acid
The production of tartaric acid plays an important role in the development of chemistry. It is believed that the first experiments on obtaining tartaric acid were carried out by the alchemist Jabir ibn Hayyan in the first century. However, the modern method of its production was developed by the Swedish chemist Karl Wilhelm Scheele only in the 18th century.
Now tartaric acid is produced from various raw materials, mainly from the waste of the wine industry. The main sources of tartaric acid production are:
- Dried wine yeast, which is obtained during the production of wine, as well as dried sediments, which are formed during the storage of sulfite wort;
- Tartar, which forms on the walls of the container during fermentation and storage of wine. As a rule, wine salts in tartar make up 60-70%;
- Tartaric lime formed during the processing of yeast, pomace, wine residues when washing barrels and other containers at many wineries;
- Cretaceous sediments that are formed in the process of reducing the acidity of wine materials and grape must with calcium carbonate.
Salts of tartaric acid - tartrates, are formed during the fermentation of grape juice.
Properties of tartaric acid
The main property of tartaric acid is considered its ability to slow down natural changes that lead to spoilage of food. In small quantities, it is not only safe for humans, but also has a beneficial effect on his body. Like natural tartaric acid, which is found in fruits, food supplement E334 has antioxidant properties and has a beneficial effect on metabolic and digestive processes in the body.
Due to these properties of tartaric acid E334 as a food additive is approved for use in the production of beverages and food in many countries of the world, which significantly increases their shelf life.
However, large doses of tartaric acid are not safe because it is a muscle toxin that can cause paralysis and death.
Application of tartaric acid

The use of tartaric acid is widespread in various industries, namely:
- Food industry as a preservative and flavor enhancer;
- Cosmetic industry, where E334 is a component of many creams and lotions for body and face;
- The pharmaceutical industry, where it is widely used in the production of various soluble medicines, as well as effervescent tablets and some other drugs;
- Analytical chemistry - for the detection of aldehydes and sugars, as well as for the separation of racemates of organic substances into isomers;
- Construction - to slow down the drying of certain building materials such as cement and gypsum;
- Textile industry - for dyeing fabrics.
The use of tartaric acid (E334) in the food industry
The main application of tartaric acid in the food industry is as an antioxidant, preservative and acidity regulator in the production of:
- Jamov;
- Ice cream;
- Table water and fizzy carbonated drinks;
- Canned food;
- Sweets;
- Various confectionery products (as an emulsifier and preservative);
- Wine;
- Jelly.
Found a mistake in the text? Select it and press Ctrl + Enter.