- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Diclofenac-AKOS
Diclofenac-AKOS: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. In case of impaired renal function
- 12. For violations of liver function
- 13. Use in the elderly
- 14. Drug interactions
- 15. Analogs
- 16. Terms and conditions of storage
- 17. Terms of dispensing from pharmacies
- 18. Reviews
- 19. Price in pharmacies
Latin name: Diclofenac-AKOS
ATX code: M01AB05
Active ingredient: diclofenac (Diclofenac)
Manufacturer: JSC Sintez (Russia)
Description and photo update: 2020-17-07
Prices in pharmacies: from 20 rubles.
Buy

Diclofenac-AKOS is a non-steroidal anti-inflammatory drug (NSAID).
Release form and composition
Diclofenac-AKOS is available in the following dosage forms:
- solution for intramuscular (i / m) administration: slightly colored transparent liquid with a slight smell of benzyl alcohol (3 ml each in a 5 ml neutral glass ampoule, 5 or 10 ampoules in a cardboard box; 5 ampoules in a blister strip, in a cardboard box 1 or 2 blister packs; in the absence of a break or notch ring and a dot in the ampoules, an ampoule scarifier is placed in each pack / box);
- ointment for external use: almost white or white with a weak specific odor (30 g each in an aluminum tube, in a cardboard box 1 tube).
Each pack also contains instructions for the use of Diclofenac-AKOS.
1 ml of solution contains:
- active substance: diclofenac sodium - 25 mg;
- additional components: sodium disulfite (sodium metabisulfite), benzyl alcohol, mannitol (mannitol), water for injection, propylene glycol, 1M sodium hydroxide solution.
100 g of ointment contains:
- active substance: diclofenac sodium - 1000 mg;
- additional components: macrogol 4000 (polyethylene glycol 4000), dimethyl sulfoxide (dimexide), propylene glycol (E1520), propyl parahydroxybenzoate (E216), methyl parahydroxybenzoate (E218).
Pharmacological properties
Pharmacodynamics
Diclofenac is a non-steroidal anti-inflammatory agent with pronounced antipyretic and analgesic effects. By indiscriminately suppressing the activity of cyclooxygenase 1 and 2, the drug disrupts the metabolic processes of arachidonic acid, reduces the number of prostaglandins in the focus of inflammation.
Against the background of rheumatic diseases, the anti-inflammatory and analgesic properties of Diclofenac-AKOS provide a significant reduction in the severity of pain, a decrease in joint swelling and morning stiffness, contributing to the improvement of their functional state. In the postoperative period and with injuries, the active substance reduces inflammatory edema and relieves pain.
Pharmacokinetics
After i / m administration of the drug at a dose of 75 mg, its maximum concentration (C max) in the blood is recorded after 15-30 minutes and is 1.9-4.8 μg / ml (on average 2.7 μg / ml). 3 hours after i / m use, the plasma concentration of diclofenac is approximately 10% of the maximum level.
With external use of Diclofenac-AKOS ointment, less than 6% of the active substance is percutaneously absorbed. In the case of application to the area of the affected joint, the content of the drug in the synovial fluid exceeds that in the plasma.
The drug binds to plasma proteins by more than 99% (a significant part interacts with albumin). The metabolic transformation of the active substance is the result of single / multiple conjugation and hydroxylation with glucuronic acid. The enzyme of the cytochrome P 450 family - 2C9 (CYP2C9) - is responsible for the metabolism of diclofenac. The pharmacological activity of metabolic products is lower than that of the main substance.
The volume of distribution with i / m use of Diclofenac-AKOS - 550 ml / kg, systemic clearance - 350 ml / min. The half-life from plasma is 2 hours. Excreted by the kidneys in the form of metabolites about 65% of the administered dose and less than 1% - unchanged, the rest of the dose is excreted in the form of metabolites in the bile. Diclofenac is excreted in breast milk.
In the presence of severe renal failure (CC below 10 ml / min), the excretion of diclofenac metabolites with bile increases, but in this case, an increase in their content in the blood is not observed.
In patients with chronic hepatitis or compensated cirrhosis of the liver, the pharmacokinetic characteristics of diclofenac do not change.
Indications for use
Diclofenac-AKOS is recommended for short-term treatment of moderate pain in the following diseases:
- chronic psoriatic juvenile arthritis, rheumatoid arthritis, gouty arthritis, ankylosing spondylitis; osteoarthritis of the spine and peripheral joints (including with radicular syndrome); tendovaginitis, bursitis, diseases of periarticular tissues and other rheumatic lesions of soft tissues;
- sciatica, lumbago, neuralgia;
- post-traumatic pain syndrome, accompanied by inflammation (with sprains, bruises, overexertion);
- postoperative pain (for solution);
- inflammatory processes of the pelvic organs, including adnexitis; algodismenorrhea (for solution);
- pain syndrome caused by myalgia (for ointment);
- muscle pains of rheumatic / non-rheumatic origin (for ointment).
Diclofenac-AKOS is used for symptomatic treatment, relieving inflammation and pain at the time of use, the drug does not affect the progression of the disease.
Contraindications
Absolute:
- endogenous or mixed bronchial asthma (asthmatic triad): partial or complete syndrome of intolerance to acetylsalicylic acid or other NSAIDs, asthma attacks, polyps of the nasal mucosa (rhinosinusitis);
- pregnancy (for ointment - III trimester), lactation period;
- age up to 18 years (for solution) and up to 6 years (for ointment);
- hypersensitivity to any component of Diclofenac-AKOS or other NSAIDs.
For the solution, additionally absolute contraindications are:
- erosive and ulcerative lesions of the gastrointestinal tract (GIT) in the acute phase;
- bleeding from the gastrointestinal tract, ulcerative colitis, Crohn's disease and other inflammatory bowel lesions;
- heart failure;
- hyperkalemia;
- acute period of liver disease, severe liver failure;
- severe renal failure with creatinine clearance (CC) below 30 ml / min;
- hematopoietic disorders, hemophilia and other hemostasis disorders;
- period after the implementation of coronary artery bypass grafting.
An additional absolute contraindication for the ointment is a violation of the integrity of the skin in the area of the intended application.
Relative contraindications (use both dosage forms of Diclofenac-AKOS with caution):
- congestive heart failure;
- erosive and ulcerative lesions of the gastrointestinal tract (for the solution - without exacerbation);
- exacerbation of hepatic porphyria;
- bronchial asthma;
- elderly age.
Additional relative contraindications for Diclofenac-AKOS injections:
- cerebrovascular pathology;
- cardiac ischemia;
- arterial hypertension;
- peripheral arterial lesions;
- a significant decrease in the volume of circulating blood (BCC), including arising after a massive surgical intervention;
- edematous syndrome;
- a history of liver disease;
- renal failure (CC below 60 ml / min);
- condition after extensive surgical interventions;
- diverticulitis;
- diabetes;
- dyslipidemia;
- anemia;
- systemic connective tissue diseases;
- severe somatic diseases;
- alcoholism, smoking;
- presence of Helicobacter pylori infection;
- long-term treatment with NSAIDs;
- combination therapy with anticoagulants, glucocorticosteroids (GCS), selective serotonin reuptake inhibitors (SSRIs), antiplatelet agents.
Additional relative contraindications for Diclofenac-AKOS ointment:
- severe liver and kidney dysfunction;
- bleeding disorders (including hemophilia, bleeding tendency, prolonged bleeding time);
- I and II trimesters of pregnancy.
Diclofenac-AKOS, instructions for use: method and dosage
Solution for i / m administration
Diclofenac-AKOS solution is injected deep into the muscle.
A single dose is 75 mg (contents of 1 ampoule). If necessary, a repeated injection of Diclofenac-AKOS is allowed, but not earlier than 12 hours after the first injection.
If other dosage forms of diclofenac are used simultaneously, the maximum daily dose should be 150 mg.
The solution should not be used for more than 2 days; if further therapy is required, the patient is transferred to oral or rectal administration of diclofenac.
Ointment for external use
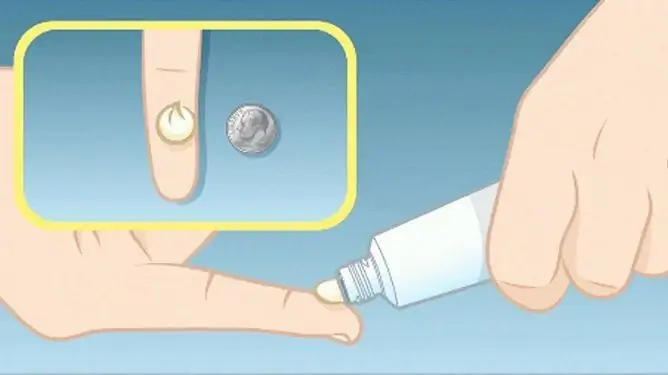
Diclofenac-AKOS ointment is used externally, applied to the skin of the painful area and rubbing lightly.
Recommended doses (with a completely open mouth of the tube part) and the frequency of applying the ointment, taking into account the age of the patients:
- children 6-12 years old: the maximum single dose is 3000 mg, which approximately corresponds to a 6 cm strip of the drug, apply no more than 2 times / day;
- adolescents over 12 years old and adults: the maximum single dose is 5000 mg, which roughly corresponds to a 10 cm long strip of the drug, the frequency of use is 2-3 times / day.
After applying the ointment, you need to wash your hands.
The course is determined depending on the indications and the observed effect. After 14 days of using Diclofenac-AKOS, you should consult a specialist.
Side effects
With the intramuscular injection of Diclofenac-AKOS solution, the following side effects from systems and organs may develop:
- nervous system: often - dizziness, headache; rarely - drowsiness; extremely rarely - impaired sensitivity, including paresthesias, tremors, convulsions, memory disorders, anxiety, irritability, depression, insomnia, disorientation, nightmares, cerebrovascular disorders, mental disorders, aseptic meningitis;
- digestive system: often - dyspepsia, flatulence, nausea, vomiting, epigastric pain, anorexia, diarrhea, increased aminotransferase activity; rarely - proctitis, gastritis, gastrointestinal ulcers (with or without bleeding / perforation), bleeding from the gastrointestinal tract (melena, vomiting with blood, diarrhea mixed with blood), jaundice, hepatitis, liver dysfunction; extremely rare - glossitis, stomatitis, constipation, damage to the esophagus, pancreatitis, diaphragmatic intestinal strictures (exacerbation of Crohn's disease or ulcerative colitis, nonspecific hemorrhagic colitis), fulminant hepatitis;
- sense organs: often - vertigo; extremely rare - tinnitus, hearing impairment, visual impairment (diplopia, blurred vision), impaired taste;
- hematopoietic organs: extremely rarely - leukopenia, thrombocytopenia, agranulocytosis, aplastic and hemolytic anemia;
- urinary system: extremely rarely - hematuria, proteinuria, nephrotic syndrome, interstitial nephritis, acute renal failure, papillary necrosis;
- cardiovascular system: extremely rarely - increased blood pressure (BP), chest pain, palpitations, heart failure, vasculitis, myocardial infarction;
- allergic reactions: anaphylactic / anaphylactoid reactions, including a significant decrease in blood pressure and shock; extremely rare - angioedema (including the face);
- respiratory system: rarely - bronchial asthma (including shortness of breath); extremely rare - pneumonitis;
- local reactions with intramuscular injection: infiltration, burning, adipose tissue necrosis, aseptic necrosis;
- skin: often - skin rash; rarely - urticaria; extremely rare - hair loss, itching, bullous rashes, photosensitivity, exfoliative dermatitis, eczema, including multiforme, and Stevens-Johnson syndrome, purpura, including allergic, Lyell's syndrome.
Adverse reactions possible with external application of Diclofenac-AKOS ointment:
- systemic effects: allergic reactions (urticaria, bronchospastic reactions, angioedema), generalized skin rash;
- local effects: photosensitization, eczema, contact dermatitis (redness, itching, swelling of the treated skin area, papules, vesicles, peeling).
Overdose
Symptoms of an overdose of diclofenac with systemic use include: headache, dizziness, shortness of breath, vomiting, blurred consciousness. Children may experience myoclonic seizures, abdominal pain, nausea, vomiting, impaired renal and liver function, and bleeding. In this condition, symptomatic treatment and forced diuresis are prescribed. During hemodialysis, diclofenac is poorly excreted from the body.
With external use, due to the very weak systemic absorption of Diclofenac-AKOS, its overdose is practically impossible. If by chance a large amount of ointment (over 20 g) was taken orally, systemic adverse events characteristic of NSAIDs may occur. In this case, gastric lavage is performed and activated charcoal is ingested.
special instructions
To reduce the threat of adverse reactions, it is recommended to use the solution in the shortest possible course in the lowest possible effective dose.
With the systemic use of Diclofenac-AKOS, it is necessary to refrain from drinking alcohol.
Do not allow ointment to be applied to open wounds. After using the product, an occlusive dressing should not be applied.
In the case of prolonged external use of the ointment in large quantities, the development of adverse systemic reactions characteristic of NSAIDs is possible.
It is necessary to avoid getting the ointment on the mucous membranes and in the eyes.
With the combined use of the ointment with other dosage forms of diclofenac, the maximum daily dose of the drug must be taken into account.
Influence on the ability to drive vehicles and complex mechanisms
During the period of treatment with the drug in the form of a solution, one should refrain from driving vehicles and working with potentially dangerous and complex mechanisms.
Application during pregnancy and lactation
The solution is contraindicated for use during pregnancy. Ointment cannot be used in the third trimester of pregnancy; in the first and second trimesters, it can only be used on the recommendation of the attending physician.
During breastfeeding, Diclofenac-AKOS therapy is not carried out.
Pediatric use
The solution is contraindicated for children and adolescents under 18 years old, ointment - for children under 6 years old.
With impaired renal function
In patients with severe renal impairment (CC below 30 ml / min), the solution is contraindicated. For moderate disorders (CC below 60 ml / min), Diclofenac-AKOS can be used under medical supervision.
Ointment for severe renal dysfunction should be administered with caution.
For violations of liver function
For patients with severe hepatic insufficiency or liver disease in the acute period, the solution is contraindicated.
Patients with a history of liver damage can be prescribed Diclofenac-AKOS solution under medical supervision.
Ointment for severe violations of the liver should be used with caution.
Use in the elderly
Elderly patients should be treated with caution with Diclofenac-AKOS.
Drug interactions
With systemic use of diclofenac, interaction reactions with the following drugs / agents are possible:
- diuretics: their effectiveness is weakened;
- thrombolytic agents (streptokinase, alteplase, urokinase), anticoagulants: the threat of bleeding increases, mainly from the gastrointestinal tract;
- potassium-sparing diuretics: increased risk of hyperkalemia;
- digoxin, cyclosporin, methotrexate, lithium preparations: the plasma level of these agents in the blood increases;
- GCS, other NSAIDs: the likelihood of adverse reactions, including bleeding from the gastrointestinal tract, increases;
- hypnotics and antihypertensive drugs: the effect of these drugs decreases;
- methotrexate: the toxicity of this substance increases;
- SSRIs, ethanol, corticotropin, colchicine, St. John's wort preparations: the risk of bleeding from the gastrointestinal tract increases;
- cyclosporine: its nephrotoxicity increases;
- antidiabetic agents: the therapeutic effect of these drugs is weakened;
- acetylsalicylic acid: the content of diclofenac in the blood decreases;
- cefoperazone, cefamandol, cefotetan, plikamycin, valproic acid: the incidence of hypoprothrombinemia increases;
- drugs leading to photosensitization: the effect of these drugs is enhanced;
- paracetamol, gold preparations, cyclosporine: the threat of the development of nephrotoxic effects of diclofenac increases;
- antibacterial agents included in the quinolone group: the threat of seizures is aggravated;
- drugs that block tubular secretion: the plasma concentration of diclofenac increases, which leads to an increase in its toxicity.
When using the ointment, it is possible to enhance the effects of drugs that cause photosensitization. With other drugs, no clinically significant interaction was found.
Analogs
Analogs of Diclofenac-AKOS are Voltaren, Diclogen, Argett Duo, Diklak, Ortofen, Naklofen, Diclofenac, Diklovit, Ortofer, Rapten Rapid, etc.
Terms and conditions of storage
Store in a place out of reach of children, protected from moisture and light. Storage temperature: solution - no higher than 25 ° C; ointment - 8-15 ° C. The solution must not be frozen.
Shelf life is 2 years.
Terms of dispensing from pharmacies
Solution for intramuscular injection is dispensed by prescription, ointment for external use - without a prescription.
Reviews of Diclofenac-AKOS
Reviews of Diclofenac-AKOS are mostly positive. Many patients find the drug an inexpensive and effective pain reliever and anti-inflammatory agent. Both dosage forms of Diclofenac-AKOS have proven themselves well in the symptomatic treatment of inflammatory and degenerative joint diseases, rheumatism, sprains and muscle sprains, sciatica and neuralgia. The solution has been successfully used for the relief of post-traumatic pain syndrome and postoperative pain. Almost all patients note the affordable cost of the drug.
The disadvantages of the remedy (mainly solution) include the development of adverse side effects. Sometimes there are reports of weak or short-term action of Diclofenac-AKOS. Some find the ointment too oily and poorly absorbed into the skin.
Price for Diclofenac-AKOS in pharmacies
The price for Diclofenac-AKOS can be:
- solution for intramuscular injection (25 mg / ml), in ampoules of 3 ml: for 5 pcs. - 18 rubles, for 10 pcs. - 35 rubles;
- ointment for external use (1%): for a tube containing 30 g - 20-40 rubles.
Diclofenac-AKOS: prices in online pharmacies
|
Drug name Price Pharmacy |
|
Diclofenac-AKOS 25 mg / ml solution for intramuscular administration 3 ml 5 pcs. RUB 20 Buy |
|
Diclofenac-AKOS 25 mg / ml solution for intramuscular administration 3 ml 10 pcs. RUB 40 Buy |
|
Diclofenac-AKOS 1% ointment for external use 30 g 1 pc. RUB 44 Buy |
|
Diclofenac-AKOS solution for intramuscular injection. 25mg / ml 3ml 10 pcs. RUB 49 Buy |

Maria Kulkes Medical journalist About the author
Education: First Moscow State Medical University named after I. M. Sechenov, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!