- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
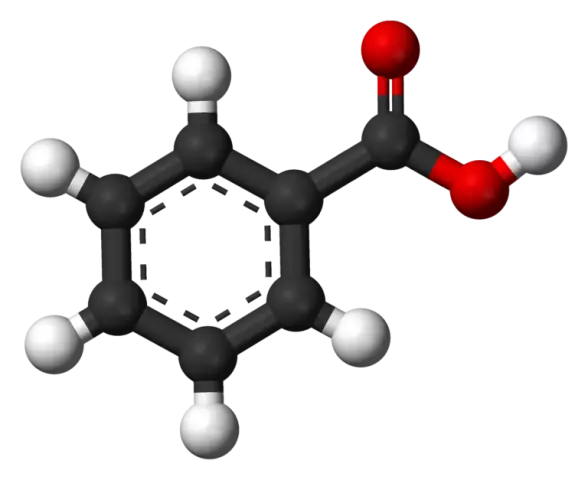
Benzoic acid
Benzoic acid (e210) is a preservative used in the food industry.
Description and characteristics

For the first time, acid was isolated in the 16th century using the method of sublimation of benzoic resin. In the 19th century, the Germans were able to determine the structure of the acid, investigated its properties and compared its characteristics with hippuric acid. As a result, in the second half of the 19th century, the antimicrobial effect of benzoic acid was revealed. And in the 20th century, it began to be widely used for preserving food.
According to its physical properties, benzoic acid is like needles or shiny monoclinic white leaves that melt at 122 degrees Celsius. The acid is readily soluble in water, fats and anhydrous ethyl alcohol.
From a chemical point of view, the preservative can be classified as an aromatic monobasic carboxylic acid. E210 is a natural substance found in a number of berries: cranberries, blueberries, lingonberries. It is found in honey in a bound form. Benzoic acid is formed in fermented dairy products such as yoghurt and yogurt, as a result of the microbial degradation of hippuric acid. It is also found in some essential oils, such as clove. The antimicrobial properties of benzoic acid are based on the inhibition of enzyme activity in microbial cells.
The acid is synthesized by oxidation of toluene. At the moment, this method of producing acid is the most common and is considered the most profitable, since the raw materials for this are inexpensive, and the process itself does not have a negative impact on the environment.
Previously, benzoic acid was also obtained by acid hydrolysis of benzotrichloride and decarboxylation of phthalic acid with the action of catalysts. But now this method of obtaining acid is not relevant.
Application of benzoic acid
In the food industry, the properties of benzoic acid are used in the confectionery, brewing and bakery industries. It is used in the preparation of margarines, jams, fruit juices, vegetable pickles, pickled fish, dairy products, chewing gum, ice cream, spices, liqueurs, sweets and sugar substitutes.

In addition, along with esters and salts, e210 is also used in the cosmetic industry. In the form of benzyl benzoate, it is used in pharmaceuticals (added to ointments against scabies).
For medicinal purposes, acid is used as a fungicidal and antimicrobial agent. It is added to many cough medicines because it acts as an antiseptic and has an expectorant effect. The e210 supplement has proven itself well in the treatment of sweaty feet and fungal skin diseases.
Benzoic acid is widely used in the chemical industry. Thus, in the synthesis of many organic substances, acid often plays the role of the main reagent.
The effect of benzoic acid on the human body
The preservative e210 is generally well absorbed by the human body and interacts with protein compounds, forming hippuric acid, in the form of which the body removes it by the kidneys.
According to some reports, e210 can interact with ascorbic acid, forming a strong carcinogen - free benzene. Therefore, products containing ascorbic acid and e210 additive should be avoided.
In Russia, there is a strictly defined dosage for the e210 preservative in food. Its amount should not exceed 5 mg / kg, otherwise the acid negatively affects the condition of the kidneys and liver.
Found a mistake in the text? Select it and press Ctrl + Enter.