- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Synflorix
Synflorix: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. Drug interactions
- 12. Analogs
- 13. Terms and conditions of storage
- 14. Terms of dispensing from pharmacies
- 15. Reviews
- 16. Price in pharmacies
Latin name: Synflorix
ATX code: J07AL52
Active ingredient: polysaccharides of Streptococcus pneumoniae (polysaccharides de Streptococcus pneumoniae)
Producer: GlaxoSmithKline Biologicals (Belgium)
Description and photo update: 28.11.2018

Synflorix is a vaccine for the prevention of diseases caused by pneumococcus.
Release form and composition
Synflorix is released in the form of a suspension for intramuscular administration: white, when standing, it is divided into two layers - a white precipitate and a colorless supernatant; after shaking - a suspension of a homogeneous consistency without conglomerates and flakes (0.5 ml in a syringe complete with a needle, in a blister of 1, 5 or 10 sets, in a cardboard box 1 blister and instructions for using Synflorix; 0.5 ml in a bottle, in carton packaging 1, 10 or 100 bottles).
Composition of 1 dose of vaccine for the prevention of pneumococcal infections (0.5 ml): conjugated with carrier proteins Streptococcus pneumoniae polysaccharides (polysaccharide serotype 4 - 3 μg / PD, serotype 19F - 3 μg / PD, serotype 18C - 3 μg / PD, serotype 14 - 1 μg / PD, serotype 23F - 1 μg / PD, serotype 9V - 1 μg / PD, serotype 1 - 1 μg / PD, serotype 5 - 1 μg / PD, serotype 6B - 1 μg / PD, serotype 7F - 1 μg / PD), carrier proteins * (D-protein of Haemophilus influenzae - 9-16 μg, tetanus toxoid - 5-10 μg, diphtheria toxoid - 3-6 μg).
Additional components: water for injection, aluminum phosphate, sodium chloride.
* The composition of the vaccine is based on the polysaccharide content. The content of the carrier protein is individual and depends on the polysaccharide / protein ratio.
Pharmacological properties
The Sinflorix vaccine contains antigens of 10 Streptococcus pneumoniae serotypes, which are most often the causative agents of invasive pneumococcal infection (50-96%) and pneumonia in children under 5 years of age.
In addition, otitis media in 60-70% of cases has a bacterial etiology and is caused by Streptococcus pneumoniae and untyped Haemophilus influenzae.
Immunological effectiveness
In clinical trials using Synflorix, an immune response was noted to all 10 serotypes contained in the vaccine, however, the magnitude of the response depended on the serotype. The immune response against serotypes 1 and 5 was slightly lower than against the rest of the serotypes. At the same time, the significance of this phenomenon for the clinical efficacy of the vaccine in the prevention of diseases caused by these serotypes has not been established.
Vaccine efficacy for the prevention of invasive pneumococcal infection
According to the requirements of the World Health Organization, the efficacy of Synflorix is assessed by comparing the immune response for 7 pneumococcal serotypes contained both in this vaccine and in the 7-valent pneumococcal conjugate vaccine with known protective efficacy (reference drug). The effect was compared according to the results of assessing the immunogenicity by the geometric mean concentrations of the formed antibodies by the enzyme-linked immunosorbent assay (ELISA) and their geometric mean titers by the method of assessing opsonophagocytic activity (OPA).
In a direct comparative study of immunogenicity, the immune response against 7 common antigens in Synflorix is comparable to that of the reference drug, with the exception of serotypes 23F and 6B (the clinical significance of this phenomenon has not been established).
The immune response was also assessed against additional serotypes 1, 5 and 7F contained in the Synflorix vaccine. Seroconversion for these antigens reached 97.3%, 99% and 99.5%, respectively.
It was also found that Synflorix induces an immune response against Streptococcus pneumoniae serotypes 6A and 19A, which are not included in the vaccine. One month after revaccination, there was an increase in the geometric mean concentrations (SGC) of antibodies to these serotypes by 5.5 and 6.1 times, respectively, and the geometric mean titers (SGT) by 6.7 and 6.1 times.
Clinical studies have confirmed the high immunogenicity of Synflorix during two-dose and three-dose primary immunization regimens in children under 2 years of age and children 2-5 years of age.
Effectiveness of the vaccine for the prevention of acute otitis media
The effectiveness of Synflorix in the prevention of acute otitis media caused not only by Streptococcus pneumoniae, but also by Haemophilus influenzae, is most likely due to the presence of D-protein in the vaccine.
The mechanism of this action of the vaccine is due to the induction of an immune response to D-protein after the initial course of vaccination with three doses during the first year of the child's life.
The vaccine efficacy for the prevention of acute otitis media was: 33.6% - disease of any etiology, 35.3% - otitis media caused by untyped Haemophilus influenzae, 35.6% - disease caused by Haemophilus influenzae (including untyped form), 51. 5% - otitis media caused by Streptococcus pneumoniae of any serotype, 65.5% - disease caused by Streptococcus pneumoniae serotypes or related serotypes contained in the vaccine (6A and 19A), 67.9% - otitis media caused by Streptococcus pneumoniae serotypes, the antigens of which are included to the composition of Synflorix.
After the end of the immunization scheme with the Sinflorix vaccine, the frequency of recurrent acute otitis media (at least 3 exacerbations after 6 months or at least 4 after 12 months) decreases by 56%, the number of episodes of catheterization of the auditory tube decreases by 60.3%.
Vaccine efficacy in a two-dose primary immunization regimen
With a two-dose scheme of primary immunization of children under 6 months of age, the value of SHT antibodies to serotypes 6B and 23F is slightly lower than that with a three-dose scheme. However, no significant differences were found between the two schemes.
The secondary immune response to revaccination in the second year of life is comparable for all serotypes, regardless of the primary vaccination schedule. Although it is worth noting that with the two-dose regimen, the antibody titer is slightly lower for serotypes 5 and 23F, the clinical significance of this phenomenon is unknown.
Thus, both schemes of primary immunization demonstrated the formation of immune memory in relation to the antigens contained in the Synflorix vaccine.
Vaccine immunogenicity in premature babies
The use of Synflorix has shown high immunogenicity in the vaccination of premature infants (born at 27-36 weeks of gestation) with three doses at 2, 4 and 6 months, followed by revaccination. In 97.6% of children, a cut-off value of FGC ≥ 0.2 μg / ml, measured by ELISA, was achieved. In 91.9% of children, opsonizing antibody (OGT) titers were ≥ 8 for all Streptococcus pneumoniae serotypes contained in the vaccine.
Thus, no fundamental differences were found in the formation of the immune response and immune memory in premature babies and babies born on time.
Indications for use
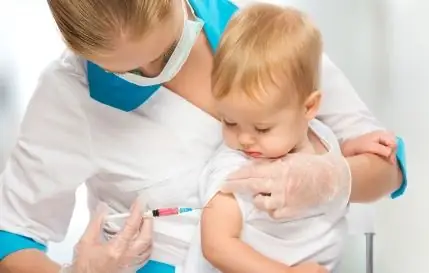
Synflorix is used for active immunization of children from 6 weeks to 5 years old for the prevention of invasive diseases (including pneumonia, meningitis, bacteremia, sepsis) and acute otitis media caused by Streptococcus pneumoniae serotypes 19F, 1, 4, 5, 6B, 7F, 9V, 14, 18C and 23F.
Contraindications
- acute diseases (both infectious and non-infectious) or exacerbation of chronic diseases *;
- hypersensitivity to vaccine components.
* These conditions are a temporary contraindication to vaccination. Synflorix can be administered 2-4 weeks after recovery from an acute illness or during remission / convalescence in case of chronic diseases. In acute intestinal diseases, mild acute respiratory viral infections, etc., the introduction of Synflorix is allowed immediately after the normalization of body temperature. If mild colds are detected, the vaccination should not be postponed.
With caution, Sinflorix (like other vaccines administered intramuscularly) should be used in patients with thrombocytopenia or other blood clotting disorders, due to the risk of bleeding during intramuscular injection.
The safety and immunogenicity of the vaccine has not been established in children with an increased risk of developing pneumococcal infections: nephrotic syndrome, sickle cell anemia, malignant neoplasms, HIV infection, congenital and acquired dysfunctions of the spleen.
Sinflorix, instructions for use: method and dosage
The suspension is administered intramuscularly only. Subcutaneous, intradermal and intravascular administration is prohibited.
Recommended vaccination sites: in children of the first year of life - the anterolateral surface of the thigh, in children over 1 year old - the deltoid muscle of the shoulder.
Immediately before administration, the vaccine must be shaken well to form a homogeneous white suspension without conglomerates and flakes. If the suspension looks different, you cannot use it.
One syringe / vial contains 0.5 ml of suspension, which corresponds to one single dose of the vaccine.
Vaccination schedules for children 6 weeks to 6 months (inclusive):
- primary three-dose immunization (preferred): the optimal age of the first vaccination is 2 months, the subsequent ones - at intervals of at least 1 month. If necessary, the earlier introduction of the first dose is possible, but not earlier than 6 weeks of the child's life. Revaccination is carried out at least 6 months after the third dose of primary immunization, preferably at 12-15 months;
- primary two-dose immunization (in the framework of mass immunization): the age of the first vaccination is 2 months, the next one - after 2 months. Revaccination is carried out at least 6 months after the second dose of primary immunization.
For immunization of premature babies (but not less than 27 weeks of gestation), a three-dose regimen is recommended, followed by revaccination. The age of administration of the first dose is 2 months, the second - no earlier than 1 month later. Revaccination is carried out at least 6 months after the second dose of primary immunization.
For children who have not been vaccinated within the first 6 months of life, the following regimens are recommended, depending on the age of the child:
- 7-11 months: 2 vaccinations with a minimum interval of 1 month, revaccination - in the second year of life, but not earlier than 2 months after the second dose of primary immunization;
- 12-23 months: 2 vaccinations, with a minimum interval of 2 months. The need for revaccination has not been established;
- 2-5 years: 2 vaccinations with a minimum interval of 2 months.
If the first vaccination was given with the Sinflorix vaccine, it is recommended to take the full course with the same vaccine.
Side effects
In clinical trials of primary immunization, approximately 4500 healthy and 137 preterm infants were observed. Used about 12,800 doses of Synflorix. Approximately 3800 healthy and 116 premature infants were revaccinated with the same vaccine in the second year of life. The safety of Synflorix has also been confirmed in the observation of approximately 200 children aged 2-5 years. In all studies, this vaccine was administered at the same time as other vaccines recommended at the appropriate age. With each subsequent vaccination during the primary vaccination, there was no increase in the frequency of side effects or their severity.
It was recorded that in the primary immunization scheme, the frequency of local reactions is slightly higher in children over 12 months of age than in children under 12 months.
With the simultaneous use of whole-cell pertussis vaccines in children, a higher reactogenicity was noted.
The following side effects were most often recorded: irritability - 52.3%, redness at the injection site - 38.3%, but these reactions quickly passed. During the period of revaccination, the frequency of the described reactions slightly increased in comparison with the primary immunization and reached 55.4% and 52.6%, respectively.
Possible side effects of Synflorix:
- local and general reactions: very often (≥ 1/10) - swelling, redness and pain at the injection site, fever (rectally ≥ 38 ° C in children under 2 years of age); often (from ≥ 1/100 to 39 ° C in children under 2 years of age, ≥ 38 ° C in children 2-5 years of age); infrequently (from ≥ 1/1000 to 40 ° С * in children under 2 years of age,> 39 ° С in children 2-5 years old);
- from the immune system: rarely (≥ 1/10 000 to <1/1000) - allergic reactions (atopic dermatitis, allergic dermatitis, eczema);
- skin reactions: rarely - rashes, urticaria;
- from the digestive system and nutrition: very often - loss of appetite; infrequently - diarrhea, vomiting;
- from the respiratory system: infrequently - apnea in severely premature babies (≤ 28 weeks of gestation);
- from the nervous system and psyche: very often - irritability, drowsiness; infrequently - pathological crying; rarely - febrile and afebrile seizures.
* Reported during revaccination.
Overdose
Overdose cases have not been reported.
special instructions
Before the introduction of Synflorix, the doctor should carefully study the history of the child being vaccinated, paying particular attention to previous vaccinations and the development of side effects.
It is extremely rare that anaphylactic reactions develop, but the risk cannot be excluded, therefore, for at least 30 minutes after the Synflorix vaccination, the child should be under medical supervision in the office, where the necessary means for anti-shock therapy are provided.
Fainting is possible as a psychogenic reaction to the injection before vaccination or during the administration of the vaccine. This should be considered when choosing an injection site to avoid injury from a fall.
The safety and efficacy of Synflorix in children over 5 years of age have not been studied.
Given the potential for apnea, monitoring of respiratory function is required for 48-72 hours during the primary vaccination of preterm infants (≤ 28 weeks gestation), especially in the presence of respiratory distress syndrome. Children in this group require immunization, so the primary vaccination should not be delayed or abandoned.
Not all people vaccinated with Synflorix can show a protective immune response (this is characteristic of the administration of any vaccine).
The use of Synflorix does not guarantee the prevention of the development of diseases caused by pneumococci of other serogroups, the antigens of which are not included in its composition. Although there has been an immune response to Haemophilus influenzae D-protein, tetanus toxoid and diphtheria toxoid, Synflorix cannot replace routine vaccination against Haemophilus influenzae type b, tetanus and diphtheria. Therefore, it is recommended to adhere to the official schedule for immunization against these infections.
The prophylactic use of an antipyretic agent before or immediately after the administration of the vaccine can reduce the frequency and severity of post-vaccination febrile reactions, therefore, it can be recommended for children with a history of febrile reactions and children receiving Synflorix simultaneously with whole-cell pertussis vaccine.
A reduced level of antibody production after immunization can be observed in children with a reduced immune status, including with a genetic defect, HIV infection, immunosuppressive therapy, and other factors. The doctor makes the decision on vaccination individually, taking into account the fact that in children 12-23 months of age, a two-dose regimen may not be enough to provide adequate protection, so revaccination may be required. With an increased risk of pneumococcal infection (for example, with sickle cell anemia, asplenia, HIV infection, chronic diseases, immune disorders), children under 2 years of age are recommended to be immunized with the Synflorix vaccine, adhering to age guidelines. Children over 2 years of age may be recommended a 23-valent pneumococcal polysaccharide vaccine (the interval after the administration of the Synflorix vaccination should be at least 8 weeks).
Application during pregnancy and lactation
Synflorix is prescribed only for children, therefore, studies on the safety of the vaccine in pregnant and breastfeeding women have not been conducted.
Pediatric use
The Sinflorix vaccine is used according to indications.
Drug interactions
The prophylactic use of paracetamol as an antipyretic agent may decrease the immune response to pneumococcal vaccines. The clinical significance of this observation is unknown.
Synflorix should not be mixed in the same syringe with any other drugs.
Synflorix may not induce an adequate immune response in patients receiving immunosuppressive therapy (like other vaccines in a similar case).
The Synflorix vaccine can be given at the same time as any of the following monovalent or combination vaccines (including AaKDS-HepV-IPV / Hib and ACKDS-HepV / Hib): against chickenpox, against poliomyelitis (OPV), against measles, mumps and rubella, against rotavirus infections against hepatitis B; diphtheria-tetanus acellular pertussis (DTP), diphtheria-tetanus whole-cell pertussis (DTP), meningococcal serogroup C conjugated (conjugates CRM 197 and TT), inactivated for the prevention of poliomyelitis (IPV), for the prevention of type influenza bile (XI) infection … Different vaccines should always be given to different parts of the body!
The safety profile and immune response of concurrently administered vaccines do not change, with the exception of the immune response to inactivated polio vaccine (inactivated poliomyelitis virus type II), which has had conflicting results (seroprotection values ranged from 78-100%). The clinical significance of this phenomenon has not been established. Regardless of the type (CRM 197 or TT), the carrier protein in meningococcal conjugate vaccines did not have any negative effect when the vaccines were combined.
An increase in the immune response to tetanus and diphtheria antigens, Haemophilus influenzae type b capsular polysaccharide conjugated to tetanus toxoid was observed.
Analogs
Synflorix analogs are: Pnevmo 23, Pnevmovax 23, Prevenar, Prevenar 13.
Terms and conditions of storage
Store in a dark place, out of reach of children, at a temperature of 2-8 ° C, without freezing.
The shelf life is 3 years.
Terms of dispensing from pharmacies
Dispensed by prescription.
Reviews about Synflorix
Reviews of Synflorix are mostly positive. This vaccine prevents the development of pneumococcal infection, which often causes complications, for example, after influenza in the form of conjunctivitis, otitis media, bronchitis, pneumonia, meningitis and other serious diseases. An additional advantage of the vaccine is protection against hemophilic infection, and the disadvantage is the absence of this vaccination in the mandatory vaccination calendar. Prevention of invasive diseases is only recommended, so parents have to pay for the vaccine themselves.
Of the negative reactions, an increase in the child's body temperature on the day of the introduction of Synflorix is most often mentioned.
Price for Synflorix in pharmacies
The approximate price for Synflorix for 1 syringe containing 0.5 ml of suspension (1 dose) is 1650 rubles.

Maria Kulkes Medical journalist About the author
Education: First Moscow State Medical University named after I. M. Sechenov, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!