- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Lanotan
Lanotan: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. Use in the elderly
- 12. Drug interactions
- 13. Analogs
- 14. Terms and conditions of storage
- 15. Terms of dispensing from pharmacies
- 16. Reviews
- 17. Price in pharmacies
Latin name: Lanotan
ATX code: S01EE01
Active ingredient: latanoprost (Latanoprost)
Manufacturer: PJSC "Farmak" (Ukraine)
Description and photo update: 2020-18-08

Lanotan is an anti-glaucoma drug, a synthetic analogue of prostaglandin F 2α for topical use in ophthalmic practice.
Release form and composition
Dosage form - eye drops: colorless transparent liquid (2.5 ml in polyethylene bottles with a self-adhesive label, sealed with a dropper with a screw-on polyethylene lid equipped with an opening control; in a cardboard box 1 bottle and instructions for use of Lanotan).
Composition for 1 ml of solution:
- active substance: latanoprost - 0.05 mg (in terms of 100% substance);
- auxiliary components: benzalkonium chloride - 0.2 mg; sodium chloride - 4 mg; sodium dihydrogen phosphate monohydrate - 4.5 mg; anhydrous sodium hydrogen phosphate - 4.7 mg; water for injection - up to 1 ml.
Pharmacological properties
Pharmacodynamics
The active component of Lanotan eye drops is latanoprost, an analogue of prostaglandin F 2a, a selective agonist of the prostanoid FP receptor, which reduces intraocular pressure by improving the outflow of aqueous humor. In humans, the process of lowering intraocular pressure begins approximately 3-4 hours after instillation of the drug; the maximum effect is observed after 8-12 hours. The antihypertensive effect of drops lasts at least 24 hours.
Based on the results of baseline studies, latanoprost has been found to be effective as monotherapy. At the same time, clinical studies of its use as part of a complex treatment were carried out, which proved the good therapeutic effect of latanoprost in combination with β-blockers, for example, timolol. In the course of short-term observations lasting from 1 to 2 weeks, the additive effect of latanoprost in combination with oral carbonic anhydrase inhibitors (acetazolamide), adrenergic agonists (epinephrine dipivalil) and, at least partially additive, in the case of combined use with cholinomimetic agents) (pilocarpine) was confirmed …
During clinical studies, no significant effect of latanoprost on the formation of aqueous humor in the eye chambers and on the blood-ophthalmic barrier was found.
In the case of short-term therapy, latanoprost does not induce the penetration of fluorescein into the posterior segment of the eye in pseudophakia.
No significant pharmacological effect of latanoprost used in clinical doses on the respiratory and cardiovascular systems has been identified.
Pharmacokinetics
Latanoprost is a prodrug with a molecular weight of 432.58 Da (dalton) esterified with an isopropyl group and does not exhibit pharmacological activity.
The prodrug penetrates well through the cornea and, like all substances that enter the intraocular fluid when passing through the cornea, undergoes hydrolysis. In the process of hydrolysis in the cornea of the eye under the action of esterase enzymes, the biologically active acid latanoprost is formed.
The volume of distribution (V d) of latanoprost is from 0.14 to 0.18 l / kg. After topical application of drops, latanoprost acid in the aqueous humor of the eye is determined during the first 4 hours, and in plasma - only during the first hour.
In the course of a study of the pharmacokinetics of latanoprost in humans, it was found that it reaches its maximum concentration (C max) in the intraocular fluid approximately 2 hours after instillation into the conjunctival sac. In primates, distribution of latanoprost after topical application occurs mainly in the anterior segment of the eye, in the conjunctiva and eyelids. The substance reaches the posterior segment of the eye in a small amount.
Latanoprost acid is practically not metabolized in the eye, the main biotransformation occurs in the liver. Entering the systemic circulation, it is metabolized by β-oxidation of fatty acids in the liver, forming 1,2-dinor and 1,2,3,4-tetranor metabolites.
The half-life (T 1/2) from plasma in humans reaches ~ 17 minutes. Systemic clearance is ~ 7 ml / min / kg. After β-oxidation in the liver, metabolites are predominantly subjected to renal elimination, so as a result of topical application in the urine ~ 88% of the dose received is excreted.
Pharmacokinetics of latanoprost in pediatric patients
In comparison with adult patients, the exposure of latanoprost in children aged 3-12 is approximately 2 times higher, in children under 3 years of age - 6 times. At the same time, the safety profile of the drug in children and adults does not differ. For patients of all age groups, the time to reach the maximum plasma concentration (T Cmax) for latanoprost acid is 5 minutes. In children, T 1/2 is identical to that in adults. At an equilibrium concentration of latanoprost acid in the blood, its cumulation does not occur.
Indications for use
Lanotan is indicated for use in order to reduce increased intraocular pressure in the following cases:
- adult patients: open-angle glaucoma and increased intraocular pressure;
- pediatric patients: childhood glaucoma and increased intraocular pressure.
Contraindications
Absolute:
- the neonatal period and infancy (from birth to 1 year) - due to the fact that there is no data on the effectiveness and safety of the drug in this age period;
- pregnancy and breastfeeding;
- established hypersensitivity to any components in Lanotan eye drops.
When using the drug, caution should be exercised in patients with the following pathologies:
- aphakia; pseudophakia with violation of the integrity of the posterior capsule of the lens; the presence of established risk factors for macular edema (cases of macular edema, including cystic edema, are known when latanoprost is used);
- inflammatory, neovascular or congenital glaucoma (there is no sufficient experience in using the drug in the treatment of these diseases).
Lanotan, instructions for use: method and dosage
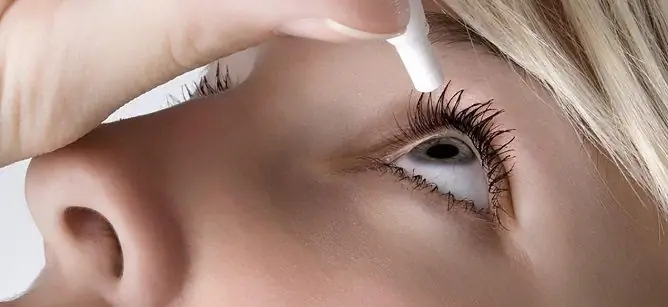
Lanotan eye drops are intended for topical application by instillation into the conjunctival sac of the affected eye (s).
For adults, including the elderly, it is recommended to instill the drug once a day, 1 drop into the affected eye. Optimum efficiency is achieved when Lanotan is used in the evening.
You should not use the drug more than once a day, since it has been proven that frequent use of drops reduces the effectiveness of reducing intraocular pressure.
If the current dose is missed, the therapy is continued, having carried out the next instillation the next day at the set time.
As with any other eye drops, in order to reduce possible systemic absorption during instillation, immediately after instillation of each drop, it is recommended to press with fingers on the lacrimal sac in the medial corner of the eye (for occlusion of the lacrimal openings) and hold for 1 min.
It is required to remove contact lenses immediately before the procedure; they can be installed again after 15 minutes.
If it is necessary to use several topical ophthalmic drugs, it is important to observe an interval of at least 5 minutes between their use.
For the treatment of children, Lanotan eye drops are used in compliance with the same dosage regimen as in adult patients.
Side effects
The use of latanoprost most often causes negative side effects on the part of the organ of vision. When conducting an open study of its effect, lasting 5 years, a change in the pigmentation of the iris was recorded in 33% of patients. Other unwanted ophthalmic reactions were usually temporary and manifested immediately after the instillation of eye drops.
The scale for determining the frequency of adverse events by the number of registered cases: very often (≥ 0.1); often (≥ 0.01, but <0.1); infrequently (≥ 0.001, but <0.01); rarely (≥ 0.0001, but <0.001); extremely rare (<0.0001); with an unspecified frequency (based on the available data, it is not possible to estimate the frequency of side effects).
When using Lanotan eye drops in adults and elderly patients, the following negative effects from systems and organs may develop:
- infections and invasions: with an unknown frequency - herpetic keratitis;
- CNS (central nervous system): with an unknown frequency - dizziness, headache;
- organ of vision: very often - hyperpigmentation of the iris of the eye, hyperemia of the mucous membrane of the eye (conjunctiva), irritation of the eye of mild / moderate severity (feeling of sand in the eyes, burning, itching, tingling, sensation of a foreign body); thickening, lengthening, change in pigmentation, an increase in the number of eyelashes; often - temporary punctate corneal erosion (mostly asymptomatic), eye pain, blepharitis, photophobia; infrequently - dry eye syndrome, eyelid edema, conjunctivitis, keratitis, blurred vision; rarely - iritis or uveitis (mainly in predisposed patients), corneal edema, macular edema, periorbital edema, corneal erosion, eyelid skin reactions, darkening of the eyelid skin, thickening / darkening / lengthening of eyelashes, change in the direction of eyelash growth, distichiasis; extremely rare - changes in the area of the eyelashes and in the periorbital area,leading to a deepening of the upper orbital-palpebral groove; with an unknown frequency - an iris cyst. In some patients, with significant damage to the cornea, in very rare cases, corneal calcification was observed due to the use of eye drops containing phosphates;
- cardiovascular system: extremely rarely - in patients with concomitant angina pectoris worsening of its course; with an unknown frequency - palpitations;
- respiratory system, organs of the chest and mediastinum: rarely - bronchospasm (including exacerbation of the disease in the presence of data on bronchial asthma in the anamnesis), shortness of breath;
- skin and subcutaneous fat: infrequently - skin rash; rarely - local skin reaction on the eyelids, darkening of the palpebral folds of the eyelid skin;
- musculoskeletal system and connective tissue: with an unknown frequency - arthralgia, myalgia;
- general disorders and local reactions: extremely rare - chest pain.
In children, the safety profile of Lanotan corresponded to that in adults; no new undesirable effects were found. In different subgroups of pediatric patients, short-term safety profiles were also similar. In children, more often than in adults, adverse reactions such as an increase in body temperature and nasopharyngitis are recorded.
Overdose
Symptoms of an overdose of latanoprost are irritation of the mucous membrane of the eyes and redness, no other side effects from the organ of vision were observed.
When unintentionally taking Lanotan inside, it is important to consider the following information: 2.5 ml (1 bottle) of the solution contains 125 μg of latanoprost, more than 90% of which undergoes first pass metabolism during the first passage through the liver. In healthy volunteers with intravenous (iv) infusion of latanoprost at a dose of 3 μg / kg, no symptoms of intoxication were recorded, but with the introduction of 5.5-10 μg / kg, abdominal pain, nausea, dizziness, increased fatigue, and sudden coming hot flashes (hot flashes), hyperhidrosis.
During experiments on monkeys with intravenous administration of latanoprost at a dose of 500 mcg / kg, no significant effect on the function of the cardiovascular system was observed. When administered intravenously, the development of transient bronchospasm was noted in monkeys.
Instillation of latanoprost into the conjunctival sac of the affected eye in patients with moderate bronchial asthma at a dose seven times the therapeutic dose did not cause bronchospasm.
In case of an overdose of Lanotan, symptomatic and supportive therapy is prescribed.
special instructions
The use of eye drops containing latanoprost can change the color of the iris over time by increasing the brown pigment in it. Before starting the course, patients should be notified of the likelihood of irreversible changes in eye color. If the drug is used to treat only one eye, irreversible heterochromia may develop.
The color of the iris of the eye mainly changed with its natural uneven pigmentation - in people with yellow-brown, brown-blue, green-brown or gray-brown eyes. In the course of studies of latanoprost, it was found that in most cases, darkening of the iris began at the beginning of treatment (the first eight months), rarely during the second or third year of therapy, and was not recorded after four years of treatment. The progression of pigmentation decreased over time and after five years it stabilized; there is no evidence of an increase in staining over five years. The safety of latanoprost has been studied in an open-label study for five years. As a result, it was found that iris pigmentation developed in 33% of patients and in most of them it was insignificant, in many cases it was not determined clinically. In people with heterogeneous iris pigmentation, the incidence ranged from 7 to 85%, with the highest prevalence in the yellow-brown iris. Variability in patients with uniform blue pigmentation was not observed, occasionally changes in the iris coloration of uniformly green, gray and brown colors were noted.
The change in the coloration of the iris is due to an increased content of melanin pigment in stromal melanocytes, and not an increase in the number of melanocytes producing it. Characterized by the appearance of brown pigmentation around the pupil and its concentric distribution to the periphery, in which, over time, the entire iris or its parts becomes brown. No further pigmentation was noted after completion of therapy. According to the available data from clinical studies, the change in eye color is not associated with any symptoms or pathological disorders in the human body.
Lanotan does not affect nevi and lentigo located on the iris of the eye. According to the results of 5-year clinical trials, there was no accumulation of pigment in the trabecular meshwork, adjacent sclera and corneal stroma, or other parts of the anterior chamber of the eye.
It has been established that the darkening of the iris does not lead to the development of undesirable clinical consequences, therefore, if it appears, therapy with latanoprost can be continued. In this case, patients must be provided with regular medical supervision; depending on the clinical picture, the course can be completed.
The experience of using Lanotan eye drops in patients with pseudophakia in the treatment of congenital, angle-closure / open-angle, pigmented forms of glaucoma is limited.
There is no information on the use of the drug for the treatment of neovascular glaucoma and secondary inflammatory glaucoma.
Latanoprost does not affect pupil size. But since there is no experience of its use in the treatment of acute attacks of angle-closure glaucoma, Lanotan should be used with caution in such patients.
Information on the use of latanoprost in the postoperative period after cataract extraction is limited, which requires caution when treating this category of patients.
Caution must be exercised when using latanoprost to treat patients with a history of herpetic keratitis. Patients with acute herpetic keratitis, as well as in the presence of anamnestic data on chronic recurrent herpetic keratitis, should avoid the appointment of Lanotan.
During therapy with latanoprost, the appearance of macular edema, including cystic edema, was noted, primarily in patients with aphakia, pseudophakia, RZKH (rupture of the posterior lens capsule), or in the presence of risk factors for the formation of cystic macular edema (in particular in patients with diabetic retinopathy and occlusion retinal veins). If necessary, the appointment of Lanotan to such patients should be used with caution.
The drug should be used with caution if there are risk factors for the development of iritis or uveitis.
The experience of using Lanotan drops in patients with bronchial asthma is limited, but in the post-registration period, in some cases, an exacerbation of asthma and / or shortness of breath was observed. This category of patients is recommended to use the drug with caution.
Cases of darkening of the skin of the periorbital region have been recorded; in a number of patients they were reversible in cases of continued use of latanoprost.
Latanoprost over time is able to cause reversible changes in eyelashes and vellus hair (thickening, lengthening, increased pigmentation, increased density, change in the direction of eyelash growth), which disappear after stopping treatment.
Benzalkonium chloride, often added as a preservative to ophthalmic drugs, is part of Lanotan. It can cause eye irritation, toxic ulcerative and / or punctate keratopathy, and is adsorbed by soft contact lenses, discoloring them.
With prolonged use of latanoprost for the treatment of patients with dry eye syndrome or other diseases of the cornea, careful monitoring of the condition is required.
Patients wearing contact lenses should remove them before instilling Lanotan eye drops. You can put on the lenses again no earlier than 15 minutes after the procedure.
The standard method of therapy for primary congenital glaucoma in children from birth to 3 years of age is currently surgical intervention through a goniotomy or trabeculotomy.
Influence on the ability to drive vehicles and complex mechanisms
In the event of a temporary loss of clarity of visual perception due to the use of Lanotan eye drops, until vision is fully restored, one should refrain from driving a car, operating complex equipment, working with machine tools and potentially hazardous production equipment.
Application during pregnancy and lactation
The safety of local use of Lanotan in pregnant women has not been established. It is known that latanoprost can have a toxic effect on the course of pregnancy, intrauterine development of the fetus and the health of the newborn. It is contraindicated to use the drug during gestation.
Latanoprost, as well as its metabolites, are able to pass into breast milk. Therefore, Lanotan therapy is contraindicated during lactation. In case of a justified need to use the drug while breastfeeding, natural feeding should be stopped or interrupted for the duration of treatment.
Animal studies did not reveal the effect of latanoprost on male and female fertility.
Pediatric use
In pediatrics, Lanotan eye drops are used to treat children over 1 year old in compliance with the dosage regimen for adults.
Information on the efficacy and safety of using the drug in the pediatric age group under 1 year is extremely limited. There are no data available on the treatment of preterm infants born before 36 weeks of gestation.
In pediatric patients from birth to 3 years of age with visual impairment, mainly due to primary congenital glaucoma, surgical intervention by trabeculotomy or goniotomy remains the therapy of choice.
The safety of long-term use of Lanotan in the treatment of children has not been established.
Use in the elderly
Elderly patients do not need to adjust the dosage regimen of Lanotan.
Drug interactions
There are no comprehensive data on the pharmacological interaction of latanoprost with other drugs / agents.
There have been reports of a paradoxical increase in intraocular pressure in the case of the combined local use of two drugs belonging to the pharmaceutical group of prostaglandin analogs. In this regard, it is not recommended to use simultaneously two or more prostaglandins, prostaglandin analogs or their derivatives.
The study of the drug interaction of latanoprost was carried out only in adult patients.
Analogs
Analogues of Lanotan are Azarga, DuoTrav, Glauprost, Glaumax, Ksalatamax, Ksalatan, Latanol, Latanoprost-Teva, Latanoprost-DIA, Latanoprost, Latanoprost-Optic, Prolatan, Trilaktan, Unilat, etc.
Terms and conditions of storage
Store at 2-8 ° С in a place protected from light. Do not freeze. Keep out of the reach of children.
After opening the bottle, the drug must be used within 42 days, stored at a temperature not exceeding 25 ° C.
The shelf life of Lanotan is 2 years.
Terms of dispensing from pharmacies
Dispensed by prescription.
Reviews about Lanotan
Reviews about Lanotan are rarely left, but they are all positive. Patients claim that with age-related deterioration in visual perception due to glaucoma, daily use of these eye drops stabilizes the level of vision.
It is important to bear in mind that Lanotan can only be used as directed by a doctor, since there are different types of glaucoma, and improperly selected drops can worsen the condition.
Price for Lanotan in pharmacies
The current price of Lanotan, eye drops 0.005%, currently ranges from 417 rubles. for 1 bottle of 2.5 ml.

Maria Kulkes Medical journalist About the author
Education: First Moscow State Medical University named after I. M. Sechenov, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!