- Author Rachel Wainwright wainwright@abchealthonline.com.
- Public 2023-12-15 07:39.
- Last modified 2025-11-02 20:14.
Dorzolamid-SOLOpharm
Dorzolamid-SOLOpharm: instructions for use and reviews
- 1. Release form and composition
- 2. Pharmacological properties
- 3. Indications for use
- 4. Contraindications
- 5. Method of application and dosage
- 6. Side effects
- 7. Overdose
- 8. Special instructions
- 9. Application during pregnancy and lactation
- 10. Use in childhood
- 11. In case of impaired renal function
- 12. For violations of liver function
- 13. Use in the elderly
- 14. Drug interactions
- 15. Analogs
- 16. Terms and conditions of storage
- 17. Terms of dispensing from pharmacies
- 18. Reviews of Dorzolamide-SOLOpharm
- 19. Price in pharmacies
Latin name: Dorzolamide-SOLOpharm
ATX code: S01EC03
Active ingredient: dorzolamide (Dorzolamide)
Manufacturer: Grotex, LLC (Russia)
Description and photo update: 2019-11-07

Dorzolamide-SOLOpharm is an antiglaucoma agent.
Release form and composition
The drug is produced in the form of eye drops: a clear liquid, almost colorless or colorless, somewhat viscous (0.4 ml each in a dropper tube made of polypropylene or low density polyethylene, 5 or 10 dropper tubes in a foil film bag or without it, in carton pack 2, 4, 6, 12, 18 bags with 5 dropper tubes, or 1, 2, 3, 6, 9 bags with 10 dropper tubes, or 10, 20, 30, 60, 90 dropper tubes; each 5, 7 or 10 ml in a plastic bottle, complete with a drop dispenser, in a cardboard box 1 bottle and instructions for use of Dorzolamid-SOLOpharm).
1 ml drops contain:
- active substance: dorzolamide hydrochloride - 22.26 mg, which corresponds to dorzolamide in the amount of 20 mg;
- additional components: sodium hyaluronate, sodium citrate dihydrate, water for injection, mannitol, 1 M sodium hydroxide solution.
Pharmacological properties
Pharmacodynamics
Dorzolamide hydrochloride is an antiglaucoma drug that belongs to the group of inhibitors of the enzyme carbonic anhydrase (CA) II. Suppression of CA in the ciliary body of the eyeball reduces the production of intraocular fluid, presumably as a result of slowing down the production of bicarbonate ions and their further reduction to sodium and fluid excretion. Thanks to this, a decrease in intraocular pressure (IOP) is achieved.
Pharmacokinetics
In the case of long-term use of the drug, its selective accumulation in erythrocytes is noted, due to selective binding to CA-II, while the plasma level of free dorzolamide remains extremely low.
The active substance forms only one metabolite, N-desethyl-dorzolamide, which inhibits the enzymes KA-II and KA-I to a lesser extent than dorzolamide. Accumulating in erythrocytes, the metabolite communicates mainly with KA-I. Dorzolamide binds to plasma proteins by about 33%.
The active substance and its metabolite are excreted mainly unchanged by the kidneys. After the completion of therapy, dorzolamide is unevenly washed out of erythrocytes, that is, in the initial phase very intensively, leading to a rapid and pronounced decrease in the concentration level, and in the subsequent - slowly, with a half-life (T ½) of about 4 months.
Indications for use
In adult patients, Dorzolamid-SOLOpharm is recommended for use in the following diseases:
- primary open-angle glaucoma;
- pseudoexfoliative glaucoma;
- ophthalmic hypertension;
- secondary glaucoma (without block of the anterior chamber angle).
In children aged 1 week and older, the drug is prescribed for the treatment of glaucoma when monotherapy is carried out or as an addition to treatment with β-blockers.
Contraindications
Absolute:
- chronic renal failure (CRF);
- pregnancy and lactation;
- age less than 1 week;
- hypersensitivity to any component of the drug.
In the presence of severe hepatic insufficiency, Dorzolamid-SOLOfarm is recommended to be used with extreme caution.
Dorzolamid-SOLOfarm, instructions for use: method and dosage
When carrying out instillations, the recommended dosage is 1 drop in the affected eye (or in both eyes) 3 times a day - morning, afternoon and evening.
In case of replacing any antiglaucoma drug with Dorzolamid-SOLOpharm, it is required to start therapy with the last one from the next day after the previous drug was discontinued.
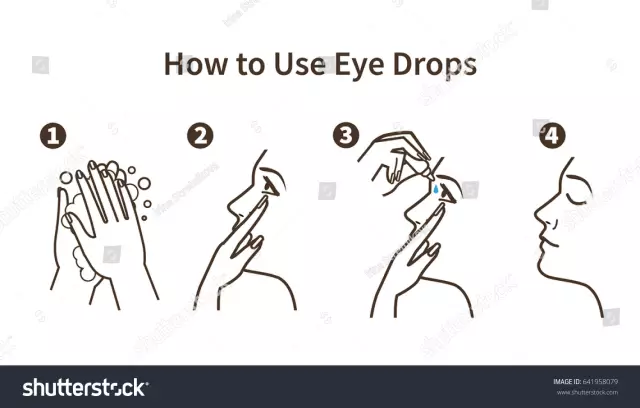
The procedure for using a dropper tube:
- Separate one dropper tube.
- After making sure that the solution is in the lower part of the dropper tube, open it - by rotating movements, turn and separate the valve.
- Drop the required dose of the solution into the eyes.
The amount of Dorzolamide-SOLOfarm eye drops contained in the dropper tube is sufficient for instillation into both eyes. After a single use, the dropper tube must be discarded, even if the drug remains in it.
Elderly patients, due to a possible hypersensitivity to dorzolamide, may require a dose reduction.
Side effects
In clinical trials, an eye drop agent containing dorzolamide was used in 1108 patients as monotherapy or as an adjunct to β-blocker therapy. In about 3% of patients, the drug was discontinued due to local ocular side effects, the most common of which were eyelid disorders and conjunctivitis.
Below are the adverse reactions recorded during the research and during the post-registration period (classified as follows: very often - ≥ 1/10, often - ≥ 1/100 and <1/10, infrequently - ≥ 1/1000 and <1/100, rarely - ≥ 1/10 000 and <1/1000, very rarely - <1/10 000, including isolated cases):
- organ of vision: very often - pain, burning; often - eyelid irritation, itching, inflammation of the eyelids, lacrimation, blurred vision, superficial punctate keratitis, conjunctivitis; infrequently - iridocyclitis; rarely - redness of the eyes, hyperkeratosis of the eyelids, transient myopia (passing after discontinuation of therapy), decreased IOP, corneal edema, detachment of the choroid after surgical interventions in order to restore the outflow of intraocular fluid;
- gastrointestinal tract: often - bitter taste in the mouth, nausea; rarely - dry mouth;
- nervous system: often - headache; rarely - dizziness, paresthesia;
- respiratory, chest and mediastinal organs: rarely - nosebleeds, pharyngitis;
- skin and subcutaneous tissue: rarely - contact dermatitis, toxic epidermal necrolysis, Stevens-Johnson syndrome;
- urinary tract: rarely - urolithiasis;
- general disorders and disorders at the injection site: often - fatigue, asthenia; rarely - allergic reactions, including systemic allergic reactions, including itching, rash, urticaria, difficulty breathing, angioedema, more rarely - bronchospasm, as well as symptoms and signs of local reactions (from the eyelids).
In the course of a three-month double-blind multicenter study of the use of the drug in children under 6 years of age, the profile of adverse reactions of dorzolamide in the form of eye drops was comparable to that in adults. The most common side effects recorded in children under 2 years of age were discharge from the eyes and conjunctival injection, in children 2-6 years old - a burning sensation / pain in the eye, inflammation of the eyelids, conjunctival injection.
Overdose
Symptoms of an overdose of Dorzolamide-SOLOpharm may include the following disorders: drowsiness, weakness, nausea, headache, dizziness, unusual dreams, electrolyte disturbances, metabolic acidosis, dysphagia. In this condition, symptomatic treatment is prescribed, aimed at maintaining the most important functions of the body. It is required to monitor plasma electrolyte concentrations (primarily potassium) and blood pH values.
special instructions
If necessary, the simultaneous use of Dorzolamid-SOLOfarm with other eye drops, instillations should be carried out at intervals of at least 10 minutes.
Influence on the ability to drive vehicles and complex mechanisms
During the period of therapy, it is not recommended to drive a car and other complex and potentially dangerous mechanisms.
Application during pregnancy and lactation
The use of Dorzolamid-SOLOfarm eye drops in pregnant and breastfeeding women is contraindicated.
Pediatric use
In newborns under 1 week of age, the use of Dorzolamide-SOLOpharm is contraindicated.
With impaired renal function
In the presence of chronic renal failure, drug therapy is contraindicated.
For violations of liver function
Patients with severe hepatic insufficiency should use Dorzolamid-SOLOpharm with extreme caution, since the safety of drug therapy in this category of patients has not been studied.
Use in the elderly
In elderly patients, an increased sensitivity to dorzolamide is possible, and therefore a dose reduction may be required.
Drug interactions
Special studies studying the interaction of Dorzolamide-SOLOfarm with other drugs / agents have not been conducted.
Possible interaction reactions with the combined use of dorzolamide as an active component of eye drops with other drugs / agents:
- eye drops containing betaxolol or timolol, systemic drugs [calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, non-steroidal anti-inflammatory drugs (NSAIDs), including acetylsalicylic acid], diuretics, hormones (insulin, estrogen, thyroxine): no negative manifestations between drugs interactions, however, it should be borne in mind that when using high doses of acetylsalicylic acid, increased toxicity may be observed;
- KA inhibitors for oral administration: mutual enhancement of the systemic effects of the drug and KA inhibitors is possible;
- local inhibitors of CA: in clinical trials, drug interactions have not been studied.
Dorzolamide-SOLOpharm belongs to CA inhibitors and, despite the fact that the drug is applied topically, it is partially absorbed and may exhibit systemic effects. In clinical trials, the use of drugs with dorzolamide in the form of eye drops did not cause disturbances in the acid-base balance, but such phenomena were observed during treatment with other CA inhibitors. Similar side effects also occurred due to interactions with other agents, as a result of toxicity in the case of therapy with high doses of salicylates. When using Dorzolamid-SOLOfarm, it is necessary to remember about the risk of developing similar reactions.
During the period of therapy, the attending physician must inform about all drugs that are used or will be used in the future (including non-prescription drugs).
Analogs
Analogs of Dorzolamid-SOLOpharm are: Dorzopt, Glaucopt, Dorzolamid-native, Trusopt, Dorzolan solo, Dor Antiglau ECO, etc.
Terms and conditions of storage
Store out of the reach of children at a temperature not exceeding 25 ° C.
Shelf life is 2 years.
Terms of dispensing from pharmacies
Dispensed by prescription.
Reviews about Dorzolamide-SOLOpharm
According to very rare reviews about Dorzolamide-SOLOpharm, found on medical sites, the drug shows good results when used according to indications. Patients note that the eye drops effectively reduce IOP and help in the treatment of glaucoma. At the same time, in almost all reviews, patients express dissatisfaction with plastic bottles equipped with a drop dispenser and tube-droppers containing the drug, since many do not know the procedure for working with these packages and consider them extremely inconvenient.
Price for Dorzolamid-SOLOpharm in pharmacies
The price of Dorzolamid-SOLOpharm, eye drops in a bottle with a drip dispenser (20 mg / ml), can be 274-394 rubles. for 5 ml, 450-465 rubles. - for 7 ml, 605-680 rubles. - for 10 ml.

Maria Kulkes Medical journalist About the author
Education: First Moscow State Medical University named after I. M. Sechenov, specialty "General Medicine".
Information about the drug is generalized, provided for informational purposes only and does not replace the official instructions. Self-medication is hazardous to health!